Table 5.
Diyne scope in the asymmetric addition to aldehydesa
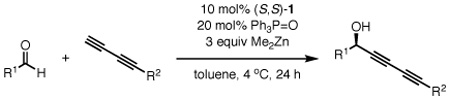 | |||||
|---|---|---|---|---|---|
| entry | aldehyde | R2 | product | % yieldb | % eec |
| 1 | 3a | 76 | 65 | ||
| 2 | 3b | 73 (49)d | 82 (79) d | ||
| 3d | 7a | 93 | 97 | ||
| 4e | 7b | 85 | 88 | ||
| 5e | 7c | 90 | 87 | ||
| 6 | 7d | 98 | 91 | ||
| 7 | 7e | 77 | 73 | ||
| 8 | 7f | >99 | 88 | ||
Reaction performed with 1 equiv of aldehyde and 3 equiv of diyne donor.
Isolated yields.
Enantiomeric excess determined by chiral HPLC.
Reaction was performed with 2 equiv of diyne and Me2Zn.
Catalyst loading was increased to 20 mol% 1 and 40 mol% TPPO.
