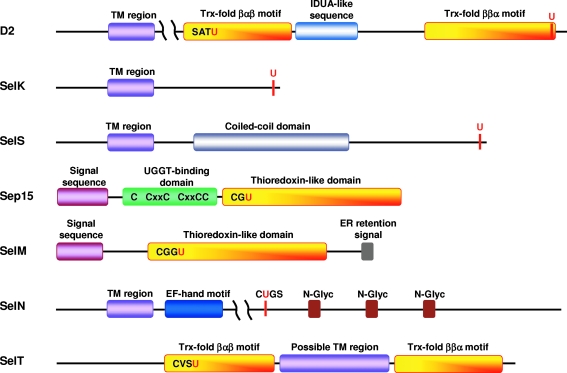
FIG. 1.
Domain organization of ER-resident mammalian selenoproteins. D2, SelK, SelS, and SelN are single-spanning membrane proteins containing an N-terminal transmembrane (TM) region (violet). The TM region begins 20 to 25 amino acids downstream from the initiator Met residue for D2, SelK, SelS, and SelN. A possible TM region of SelT also is shown in violet. Thioredoxin-like fold is known for D2 [βαβ motif (42 aa) and ββα motif (41 aa)], Sep15 (72 aa), SelM (72 aa), and SelT (141 aa) (yellow). The N-terminal signal peptide detected for Sep15 (32 aa) and SelM (24 aa) is shown in pink. Other annotated domains or predicted elements include the IDUA-like sequence in D2 (blue), the coiled-coil domain in SelS (colored in gray), UGGT-binding domain in Sep15 (green), the ER retention signal in SelM (grey), and EF-hand motif (dark blue) and three predicted N-glycosylated sites (residues 482, 504, and 530) in SelN (brown). Location of Sec (U) in all selenoproteins is shown in red. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).