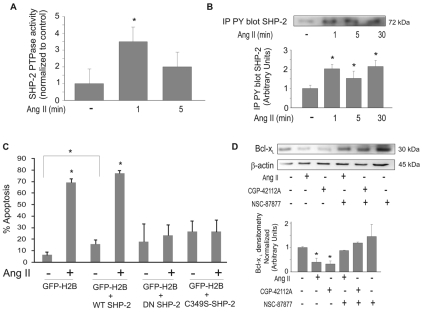
Fig. 6.
SHP-2 is activated by Ang II. (A) PAECs were treated with 10 μM Ang II for the indicated times. Cell lysates were immunoprecipitated with anti-SHP-2. Immunoprecipitated SHP-2 protein was then subjected to phosphatase assays. (B) SHP-2 phosphorylation in response to Ang II. PAECs were treated with 10 μM Ang II for the indicated times. Cell lysates were prepared and equal amounts of protein were immunoprecipitated for phospho-tyrosine and blotted for SHP-2. Graph shows densitometry of phospho-SHP-2 bands. (C) PAECs were transfected with wild-type (WT) SHP-2, dominant negative (DN) SHP-2 or the C459S-mutant phosphatase-inactive SHP-2 (C459S-SHP-2). An expression vector for EGFP-tagged H2B histone (GFP) was co-transfected as a marker. Cells were untreated or treated with 10 μM Ang II for 24 hours and used for neutral comet assays. GFP-H2B was used to visualize transfected cells and propidium iodide was added to stain comet tails. (D) Cells were treated ± SHP-1/2 phosphatase inhibitor (NSC-87877) prior to treatment with 10 μM Ang II or 10 μM AT2 agonist (CGP-42112A). Cell lysates were prepared and equal amounts of protein were used for western blotting for Bcl-xL. Blots were stripped and probed for β-actin as a loading control. Graph shows densitometry analysis of Bcl-xL normalized to β-actin. All data show means ± s.d. *Statistical significance from control, P<0.05, n=3. Experiments were repeated at least three times.