Fig. 6.
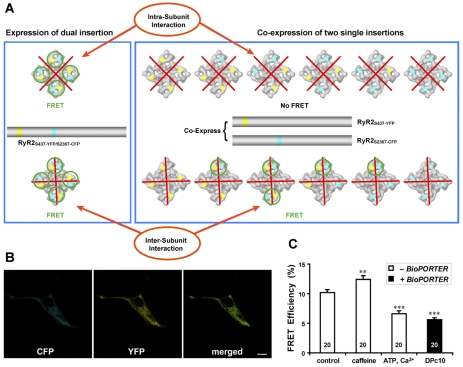
The interaction between N-terminal and central mutation regions is an inter-subunit interaction. (A) Proposed model of domain-domain interaction. The site of N-terminal YFP insertion after Ser437 (yellow dots) and the central domain CFP insertion after Ser2367 (cyan dots) are adjacent in the clamp region of RyR2, and form a domain switch that is important for channel gating. What was unclear was whether this domain switch is formed by two domains located in one subunit (top row), or from two domains belonging to two neighboring subunits (bottom row). For the cDNA construct that contains both YFP inserted after Ser437 and CFP after Ser2367, FRET is predicted when the cDNA is expressed, regardless of whether the interaction is within a subunit or between two subunits (left panel; FRET pairs indicated with green circles). We constructed two RyR2 cDNAs, one with CFP inserted after Ser437, the other with YFP inserted after Ser2367. When these two cDNAs are coexpressed in the HEK293 cells, six possible hybrid RyR2 molecules can result (right panel). The top row shows the six possible structures if the domain switch is formed by two domains contained within one subunit (i.e. an intra-subunit interaction); in this case, no FRET signal will be detected, because the distance between CFP and YFP in two separate clamp corners is over 200 Å. The bottom row shows the six possible structures if the domain switch is formed at the interface of two neighboring subunits (i.e. an inter-domain interaction); in this case, four out of six have at least one CFP-YFP pair within the same clamp region (highlighted by light green circles). FRET is predicted in these four cases, similar to what is expected (left panel), and to what we indeed observed (data shown in Fig. 3) for RyR2S437-YFP/S2367-CFP. (B) Images of live HEK293 cells co-transfected with cDNAs of RyR2S437-YFP and RyR2S2367-CFP show the colocalization of CFP and YFP. Scale bar: 10 μm. (C) The FRET efficiency as determined by the photobleaching method. FRET signals were detected in HEK293 cells, demonstrating that the domain switch formed between the N-terminal and central mutation domains is an inter-subunit interaction. The average FRET efficiency for the control cells was 10.2±0.5 (n=20 cells); for caffeine-treated cells, 12.4±0.6 (n=20), for ATP and Ca2+, 6.6±0.5 (n=20), and for cells treated with DPc10 (with BioPORTER), 5.6±0.3 (n=20). **P<0.01, and ***P<0.001, compared with control.