Abstract
Our ability to predict consequences of climate change is severely impaired by the lack of knowledge on the ability of species to adapt to changing environmental conditions. We used distribution data for 140 mammal species in Europe, together with data on climate, land cover and topography, to derive a statistical description of their realized climate niche. We then compared climate niche overlap of pairs of species, selected on the basis of phylogenetic information. In contrast to expectations, related species were not similar in their climate niche. Rather, even species pairs that had a common ancestor less than 1 Ma already display very high climate niche distances. We interpret our finding as a strong interspecific competitive constraint on the realized niche, rather than a rapid evolution of the fundamental niche. If correct, our results imply a very limited usefulness of climate niche models for the prediction of future mammal distributions.
Keywords: niche evolution, niche model, species distribution model, mammal, phylogeny, phylogenetic signal
1. Introduction
Adaptive radiation and allopatric speciation are the key mechanisms in the creation of species diversity (Schluter 2001; Gavrilets & Losos 2009). Rapid adaptation to new or altered environmental conditions has been shown experimentally (e.g. Losos et al. 1998), by analysis of palaeontological data (Thompson 1998) and by comparisons of species across phylogenies (Benton 2009; Evans et al. 2009). Up to now, speciation has been commonly viewed as arising from adaptation to different habitats (Gavrilets & Losos 2009) and isolation (Schluter 2009), but rarely to climate (but see Evans et al. 2009). It could also thus be argued that the current changing climate may not have too severe consequences for species' continued existence, because they are able to adapt and evolve at a similar pace. A key question is whether phylogenetic constraints such as potential genetic and epigenetic mechanisms that restrict the evolution of new varieties within taxa (cf. Losos 2008; Wiens 2008) may be too strong to allow adaptive shifts in climate niches. Indeed, Kozak et al. (2006) show how the conservation of climate niches can lead to geographical displacement and hence peripatric speciation.
Here, we investigate the degree to which terrestrial mammals overlap in their multidimensional climate niche. European mammals are particularly well suited for such an analysis because of three features: (i) a mammal supertree phylogeny has recently been published (Bininda-Emonds et al. 2007); (ii) a reliable database of mammal distributions within Europe (Temple & Terry 2007) is available; and (iii) mammals are species-rich enough to yield conclusive results. Together with data on climate, land cover and topography, these data allowed us to fit species distribution models to 140 native terrestrial European mammals and calculate climate niche overlap. For each species, we compared the climate niche distance and the phylogenetic distance to its closest relative. In accordance with the hypothesis of phylogenetic signal (Losos 2008), we tested the hypothesis that closely related species also share very similar climate niches. If this hypothesis is falsified this would indicate a lack of phylogenetic niche conservatism as well (Losos 2008).
2. Material and methods
We combined three types of data in our analysis: distribution data on all European mammals (taken from Temple & Terry 2007), environmental information (climatic, topographic and land-cover data) and phylogenetic information (from Bininda-Emonds et al. 2007). Spatial data were gridded to 50 × 50 km, yielding 3037 cells from 11° to 32° E, and from 34° to 72° N. Owing to collinearity within the environmental data, we selected 13 final predictor variables from an initial set of 24 (see electronic supplementary material for a detailed description of variables and selection methods), of which five were climate variables (growing degree days over 5°C, annual precipitation, summer precipitation, temperature seasonality and residuals of absolute minimum temperature).
Distribution data were analysed using Boosted Regression Trees (BRT, following Elith et al. 2008). Across all species, climatic variables explained 56 per cent (1 s.d. = 15.5%) of the variation in species occurrences, confirming that the climatic niche played a dominant role in explaining distributional patterns. Spatial autocorrelation was present, but at a very short distance only, and could not be improved by methods presented in Dormann et al. (2007); see the electronic supplementary material. We then calculated overlap in climate niches between sister species (which were identified by cophenetic distances from the phylogenetic tree; Paradis et al. 2004). To do so, we computed predicted values from the BRTs to a five-dimensional climate dataset, which varied the five climate variables in 20 equidistant steps, but kept all other predictors at their median value. We then clipped the dataset to include only data points inside the five-dimensional convex hull of the 3037 European cells (i.e. the realized climate space). Our climate-niche dataset comprised 185 308 data points. Niche overlap (NO) was calculated on the basis of this hyperdimensional climate space (not as geographical overlap) as
where 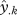 is the predicted occurrence probability for the kth of N climate hypercube combinations (normalized so that
is the predicted occurrence probability for the kth of N climate hypercube combinations (normalized so that 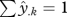 , thereby correcting for different prevalences and hence mean expected occurrence probabilities) and species i or j. This index scales predicted probabilities by the maximum of both species, yielding values from 0 to 1. For niche distance, we use 1 − NO. Using different measures of niche overlap made no difference to the outcome (see the electronic supplementary material). Finally, we used a null model to examine, whether our results were artefacts of species occupying different geographical locations and hence seemingly different climate niches. This was not the case (see the electronic supplementary material).
, thereby correcting for different prevalences and hence mean expected occurrence probabilities) and species i or j. This index scales predicted probabilities by the maximum of both species, yielding values from 0 to 1. For niche distance, we use 1 − NO. Using different measures of niche overlap made no difference to the outcome (see the electronic supplementary material). Finally, we used a null model to examine, whether our results were artefacts of species occupying different geographical locations and hence seemingly different climate niches. This was not the case (see the electronic supplementary material).
3. Results
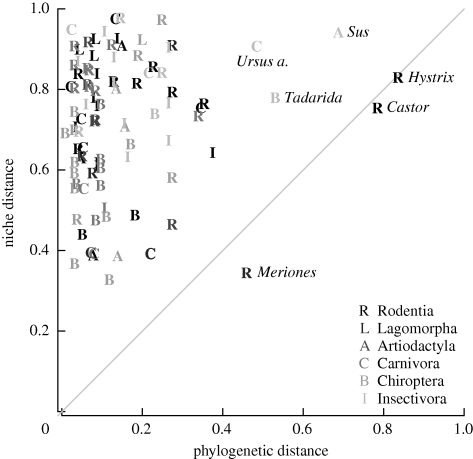
We found that closely related species differed widely with respect to their climate niche (figure 1). For the vast majority of comparisons, climate niche overlap was much smaller than would be expected from their phylogenetic relatedness (assuming constant mutation rates), hence we detected no phylogenetic signal with respect to climate niche distances of sister species. Across all species, a very weak phylogenetic trend was discernable, relating to 23 of the 140 species (21 positive, two negative trends; see the electronic supplementary material). This faint signal indicates that phylogenetic constraints were largely unimportant for the currently realized climate niche of European mammals.
Figure 1.
Climate niche distance and phylogenetic distance for a comparison of 140 mammalian sister species. Diagonal line separates niche flexible (upper left) and niche conservative (bottom right) pairs. High values for phylogenetic distance indicate species only very distantly related to any other species (e.g. crested porcupine Hystrix cristata and European beaver Castor fiber), and for niche distance, very different realized climate niches.
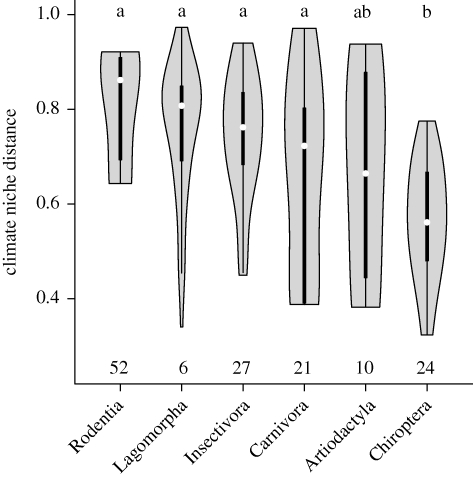
Within the lagomorpha, rodentia and insectivora, niche distances between sister taxa were significantly greater than in the chiroptera (figure 2). However, scatter was also large within groups and precluded a more in-depth analysis.
Figure 2.
Violin plot of climate niche distances for the six mammalian orders. Number of species within each group is given below each bar. Groups sharing the same letter are not significantly different in Tukey's honest significant difference post hoc test (i.e. p>0.05).
4. Discussion
Our analysis indicates high flexibility of realized climatic niches independent of phylogenetic distances. One might conclude that owing to the rapid evolution of climate niches in European mammals, climate change poses a minor threat to these species. The alternative explanation, and the more conservative one, is that the fundamental niche of the mammals investigated here is much wider than the realized niche (Kearney 2006). Competition between closely related species may have shifted the realized climate niche without requiring major evolutionary adaptations.
Apparently, climate niche space is similarly subject to character displacement as other dimensions of the niche hypervolume (size (Hutchinson 1959); (Diamond 1975); size of prey (Hespenheide 1975); forage quality (Olff et al. 2002); mutualistic gut microbe community (Ley et al. 2008); soil nutrient requirements (Tilman 1982)). Because our analysis does not comprise extinct mammal species (because both genetic and distributional data are known to a far lesser extent), we are hesitant to invoke the ‘ghost of competition past’ (Connell 1980) for the observed climate niche displacement. At the same time, we found no obvious convincing alternative explanation (e.g. shared pathogens, hybridization vigour, genetic drift; see Schluter 2001 for review).
From species ranges analysis it is known that mammals, as endothermic organisms, can occupy broader fundamental climate niches than insects or plants because they are able to buffer variation in climate (see also Gaston 2003). It is thus well conceivable that their fundamental climate niche is rather wide and less subject to physiological constraints than that of poikilothermic animals. Competition would thus simply act on the realized, not on the fundamental, climate niche. We speculate that a comparison with other species groups such as reptiles or insects should show a stronger phylogenetic signal.
European mammals have been challenged by alternating climatic conditions for several million years (DeSantis et al. 2009). The current speed of climate change is rapid, both in geological and evolutionary terms (IPCC 2007). Depending on the interpretation of our observed large difference between the phylogenetic and the climate niche signal, we may regard climate change as problematic or not. If we assume that climate niches have evolved to what we observed, then this would indicate rapid evolution. For plants (Sjöström & Gross 2006) as well as marsupials (Johnson et al. 2002), a correlation between extinction risk and phylogenetic similarity has been shown, indicating that genetic variability may not keep up with changing environments. But even the observed high degree of climate niche evolution is unlikely to suffice for European mammals to evolve in situ to climate change. The most recent speciation event documented within our dataset occurred almost 400 000 years ago (between the two bat species Rhinolophus euryale and R. mehelyi), or more than two ice ages before today. Such phylogenetic data, however, do not allow an investigation of climate niche changes within species, where most adaptation is likely to occur.
The alternative interpretation, namely that our measurement of the climate niche represents the realized rather than the fundamental climate niche, would lead to the opposite conclusion: realized climate niches bear little resemblance to the underlying fundamental niche. In this interpretation we would state that any projection of future climate change scenarios made on the basis of current distribution data alone will be misleading, because it is very likely that competition determines the niche, not the species' ability to inhabit a parameter space where it is currently not observed (see also Nogués-Bravo 2009).
Thus, while European mammals show hardly any phylogenetic signal in their climate niches, this presents no guarantee for their survival under climate change. Because mammal populations worldwide (and those in Europe are no exception) are also threatened by habitat loss, pollution and accidental mortality (Schipper et al. 2008), climate change is only one of several threats dormice and brown bears are facing.
Acknowledgements
Many thanks to Michael Holyoak, Boris Schröder and an anonymous reviewer for comments on a previous version. C.F.D. and B.G. acknowledge the Helmholtz Association for funding (VN-NG-247).
References
- Benton M. J.2009The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732 (doi:10.1126/science.1157719) [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds O. R. P., et al. 2007The delayed rise of present-day mammals. Nature 446, 507 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- Connell J. H.1980Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35, 131–138 (doi:10.2307/3544421) [Google Scholar]
- DeSantis L. R. G., Feranec R. S., MacFadden B. J.2009Effects of global warming on ancient mammalian communities and their environments. PLoS ONE 4, e5750 (doi:10.1371/journal.pone.0005750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M.1975Assembly of species communities. In Ecology and evolution of communities (eds Cody M., Diamond J. M.), pp. 342–444 Harvard, MA: Belknap Press [Google Scholar]
- Dormann C. F., et al. 2007Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 (doi:10.1111/j.2007.0906-7590.05171.x) [Google Scholar]
- Elith J., Leathwick J. R., Hastie T.2008A working guide to boosted regression trees. J. Anim. Ecol. 77, 802–813 (doi:10.1111/j.1365-2656.2008.01390.x) [DOI] [PubMed] [Google Scholar]
- Evans M. E. K., Smith S. A., Flynn R. S., Donoghue M. J.2009Climate, niche evolution, and diversification of the ‘bird-cage’ evening primroses (Oenothera, sections Anogra and Kleinia). Am. Nat. 173, 225–240 (doi:10.1086/595757) [DOI] [PubMed] [Google Scholar]
- Gaston K. J.2003Structure and dynamics of geographic ranges Oxford, UK: Oxford University Press [Google Scholar]
- Gavrilets S., Losos J. B.2009Adaptive radiation: contrasting theory with data. Science 323, 732–737 (doi:10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- Hespenheide H. A.1975Prey characteristics and predator niche width. In Ecology and evolution of communities (eds Cody M., Diamond J. M.), pp. 158–180 Harvard, MA: Belknap Press [Google Scholar]
- Hutchinson G. E.1959Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 93, 145–158 (doi:10.1086/282070) [Google Scholar]
- IPCC 2007Climate Change 2007. The physical science basis. In Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change (eds Solomon S., Qin D., Manning M., Marquis M., Averyt K., Tignor M., Miller H. L., Jr, Chen Z.), p. 996 Cambridge, UK: Cambridge University Press [Google Scholar]
- Johnson C. N., Delean S., Balmford A.2002Phylogeny and the selectivity of extinction in Australian marsupials. Anim. Conserv. 5, 135–142 (doi:10.1017/S1367943002002196) [Google Scholar]
- Kearney M.2006Habitat, environment and niche: what are we modelling? Oikos 115, 186–191 (doi:10.1111/j.2006.0030-1299.14908.x) [Google Scholar]
- Kozak K. H., Wiens J. J.2006Does niche conservatism promote speciation? A case study in North American salamanders. Evolution 60, 2604–2621 [PubMed] [Google Scholar]
- Ley R. E., et al. 2008Evolution of mammals and their gut microbes. Science 320, 1647–1651 (doi:10.1126/science.1155725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos J. B.2008Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 11, 995–1003 (doi:10.1111/j.1461-0248.2008.01229.x) [DOI] [PubMed] [Google Scholar]
- Losos J. B., Jackman T. R., Larson A., de Queiroz K., Rodríguez-Schettino L.1998Contingency and determinism in replicated adaptive radiations of island lizards. Science 279, 2115–2118 (doi:10.1126/science.279.5359.2115) [DOI] [PubMed] [Google Scholar]
- Nogués-Bravo D.2009Predicting the past distribution of species climatic niche. Global Ecol. Biogeogr. 18, 521–531 (doi:10.1111/j.1466-8238.2009.00476.x) [Google Scholar]
- Olff H., Ritchie M. E., Prins H. H. T.2002Global environmental controls of diversity in large herbivores. Nature 415, 901–904 (doi:10.1038/415901a) [DOI] [PubMed] [Google Scholar]
- Paradis E., Claude J., Strimmer K.2004APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- Schipper J., et al. 2008The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230 (doi:10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- Schluter D.2001Ecology and the origin of species. Trends Ecol. Evol. 16, 372–380 (doi:10.1016/S0169-5347(01)02198-X) [DOI] [PubMed] [Google Scholar]
- Schluter D.2009Evidence for ecological speciation and its alternative. Science 323, 737–741 (doi:10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- Sjöström A., Gross C. L.2006Life-history characters and phylogeny are correlated with extinction risk in the Australian angiosperms. J. Biogeogr. 33, 271–290 (doi:10.1111/j.1365-2699.2005.01393.x) [Google Scholar]
- Temple H. J., Terry A.2007The status and distribution of European mammals, p. 48 Luxembourg: Office for Official Publications of the European Communities [Google Scholar]
- Thompson J. N.1998Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329–332 (doi:10.1016/S0169-5347(98)01378-0) [DOI] [PubMed] [Google Scholar]
- Tilman D.1982Resource competition and community structure Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- Wiens J. J.2008Commentary on Losos (2008): niche conservatism deja vu. Ecol. Lett. 11, 1004–1005 (doi:10.1111/j.1461-0248.2008.01238.x) [DOI] [PubMed] [Google Scholar]