Abstract
Individuals often consistently differ in personalities and behaviours that allow them to cope with environmental variation. Flight initiation distance (FID) has been measured in a variety of taxa as an estimate of the risk that an individual is willing to take when facing a predator. FID has been used to test life-history trade-offs related to anti-predatory behaviour and for conservation purposes such as to establish buffer zones to minimize human disturbance, given its species-specific consistency. Individual consistency in FID, however, has been largely overlooked. Here we show that, even after controlling for several confounding effects, this behaviour has a strong individual component (repeatability = 0.84–0.92) in a bird species, leaving a small margin for behavioural flexibility. We hypothesize that individuals may distribute themselves among breeding sites depending on their individual susceptibility to human disturbance. This habitat selection hypothesis merits further research, given its implications on both evolutionary and applied ecology research. For example, selection of human-tolerant phenotypes may be promoted through the humanization of habitats occurring worldwide, and when population means instead of individual variability in FID are considered for designing buffer zones to reduce human impacts on wildlife.
Keywords: disturbance, personalities, habituation, temperaments, disturbance-induced habitat selection hypothesis
1. Introduction
An increasing body of evidence indicates that individuals consistently differ in behavioural traits such as aggressiveness, avoidance of novelty, willingness to take risks, exploration or sociality (Réale et al. 2007). This variability in behavioural tendencies can influence how individuals cope with both predictable and stochastic environmental variation and, consequently, how their populations may persist in a humanized and dynamic environment (Dall et al. 2004). Nevertheless, despite the growing literature showing the ecological significance of individual consistency in some behaviours, its application to wildlife conservation is largely overlooked (McDougall et al. 2006).
Flight initiation distance (FID), the distance between an approaching human and a focal animal at which the latter flees, provides a standardized estimate of the risk that an individual is willing to take when facing a potential predator (Blumstein 2006). FID has been measured in a variety of taxa (insects, crabs, fishes, amphibians, reptiles, mammals and birds) to test hypotheses on the evolutionary ecology of anti-predator behaviour (e.g. Stankowich & Blumstein 2005; Rodríguez-Prieto et al. 2009). Moreover, FID is considered to be a species-specific trait useful for conservation purposes, such as to establish buffer zones for minimizing human disturbance (Tarlow & Blumstein 2007). However, research on FID may be flawed by the fact that it tends to focus on central tendency rather than on individual variation. While FID has been shown to vary strongly among species (Blumstein et al. 2003) and populations (Martínez-Abrain et al. 2008), there has been only a single attempt to address the individual level, which failed to find consistent differences in FID among nine individuals (Runyan & Blumstein 2004). Here, through repeated measures of FID taken in a large population of birds, we show a high level of individual consistency in this behaviour. Thus, we provide a new conceptual framework for studies using FID and their theoretical and applied results.
2. Material and methods
The burrowing owl (Athene cunicularia) is found across American open landscapes, showing diurnal activity and nesting in burrows excavated by the owl or by mammals (del Hoyo et al. 1999). Pairs are territorial and highly conspicuous in the daylight during the breeding season, being easily located usually within 30 m of their nests. Sexual differences in coloration and plumage patterns (del Hoyo et al. 1999; M. Carrete & J. L. Tella 2006, unpublished results) allow experienced observers to sex breeding adults at a distance using binoculars.
We GPS-located 737 active nests in a 3500 km2 area of agricultural and semi-natural grasslands near Bahía Blanca, Argentina, from which we randomly selected 59 nests for this study. FIDs of adult breeders were measured by J.L.T. at the end of the nestling period (1–30 January 2009), by walking towards focal individuals in a standardized manner (following a direct trajectory, with no obstacles blocking the bird and the observer and at a constant speed of 0.5 m s−1), using a laser telemeter (8×, range:10–800 m) or counting paces for distances of less than 10 m. Owls were easily located at a distance, given the bare ground and short vegetation surrounding their nests, sited in overgrazed pasturelands. Since owls were usually ground-resting close to or at the entrance of their nests, we pre-established a starting distance (approx. 200 m) constant for all birds, thus avoiding variability in FID associated with varying starting distances (e.g. Rodríguez-Prieto et al. 2009). Successive trials (two to five per individual every 3 days) were alternatively conducted in the morning and afternoon. The number of trials per individual was constrained by the large sample size and the approximately one-month fledgling period (early January–mid-February). We are confident that we repeatedly measured FID in the same individuals because the sex of territory owners is easily recognizable by plumage differences (figure 1), and territories were at least 1 km distant from others, thus avoiding the possibility of mistaking birds.
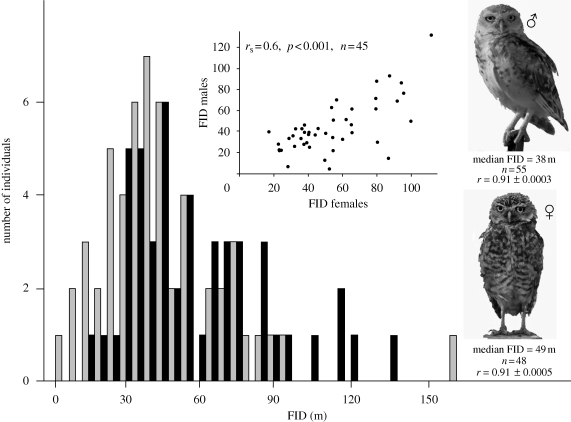
Figure 1.
FIDs of 103 burrowing owls (grey bars, males; black bars, females) measured in the first trial. Inset figure: median FID of individuals belonging to the same territory.
Despite the apparent habitat homogeneity of our study area, territories actually differ in predation pressure and distances from unpaved roads (2–170 m from nests), and we could define four subareas with different, though low-level, human activities (0.34–2.4 cars h−1, 0–0.1 pedestrians h−1; M. Carrete & J. L. Tella 2009, unpublished data). Thus, to some extent, differences in behaviour could reflect differences in predation risk and human disturbance among territories and/or subareas. Furthermore, as we measured the same individual several times, owls could become habituated to the observer. Finally, behavioural differences between sexes could also affect FIDs. Following Réale et al. (2007), we used generalized linear mixed models (GLMM) to assess individual consistency in FID (individual identity included in models as a random term) while controlling for all these potential confounding factors. In our study, mated owls belonging to the same territory have similar FID (figure 1), so individual and territory were tested as a nested (individual within territory) random term. Using the estimates of the covariance parameters, we calculated the repeatability (r) of FID within individuals and territories as the proportion of the total variability explained by each term. The s.e. of r was calculated following Becker (1984).To state that FID actually was an individual trait rather than a correlate of other sources of variability, we compared repeatability values obtained from GLMMs which controlled for sex, territory, subarea, distance to roads and the accumulated number of trials. Finally, we conducted a reaction norm approach by testing a potential interaction between individuals (random effect) and the rank of successive trials (fixed effect; Réale et al. 2007).
3. Results
We measured two or more FIDs in 103 individuals. FID greatly varied among individuals tested in a first trial (4–155 m, median: 41 m; figure 1), being highly repeatable within individuals (r = 0.92 ± 0.0002, table 1). When controlling for other sources of variability in FID, the best model reflecting to AIC values included sex of the individuals and distance from territories to roads, but within-individual repeatability remained very high (r = 0.88, individual models, table 1). Individuals’ behaviour might simply reflect the levels of predation risk and/or disturbance at their territories. However, territory models were poorly supported by AIC values and FID was much more consistent within individuals than within territories (individual versus territory models, table 1). In fact, within-individual repeatabilities remained identical when individuals nested within their territories were tested as an alternative random factor (individual (territory) model, table 1). Therefore, the moderate within-territory repeatability is a by-product of both the high repeatability within individuals holding those territories and similarity in FID between mate members (figure 1). Finally, habituation to the observer was not evident, as shown by the lack of statistical significance (p > 0.70) of the number of successive trials and its interactions with distance to roads and subareas in all models.
Table 1.
Repeatability of FIDs in 103 burrowing owls. Models test repeatability within individuals, territories, and individuals nested within territories, fitted as random terms in GLMMs. Only statistically significant models are shown.
| models |
repeatability ( ± s.e.) | AIC | |
|---|---|---|---|
| random effects | fixed effects | ||
| individual | none | 0.92 (0.0002) | −352.4 |
| sex + distance road | 0.88 (0.0004) | −392.8 | |
| sex + territory | 0.84 (0.0006) | −361.0 | |
| sex + territory(subarea) | 0.84 (0.0006) | −361.0 | |
| territory | none | 0.64 (0.0022) | −118.3 |
| sex + distance road | 0.54 (0.003) | −190.0 | |
| individual (territory) | none | 0.92 (0.0002) | −352.4 |
| sex + distance road | 0.88 (0.0004) | −392.8 | |
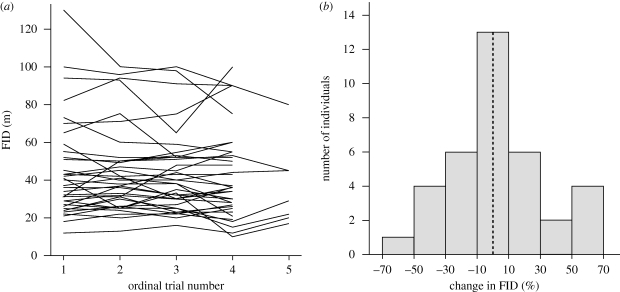
A reaction norm approach, using the subsample of 36 individuals for which we conducted four or more trials (to increase the gradient of ‘environmental variation’), again showed a strong individual component (r = 0.92 ± 0.0004) and a statistically significant interaction of individual × trials (F35,79 = 2.07, p = 0.0037; figure 2).
Figure 2.
Changes of FID across successive trials, for 36 individuals tested four or more times. (a) Individual changes. (b) Percentage change in FID between the last and the first trial.
4. Discussion
Recent studies have shown individual consistency in a variety of behavioural traits (Réale et al. 2007). To our knowledge, the only study aimed at assessing individual repeatability in FID failed to find individual consistency (Runyan & Blumstein 2004), probably owing to the low sample size (nine individuals tested three or more times), which probably did not successfully capture the variability in the study species. Here, using an adequate sample size (more than 100 individuals), we found a strong repeatability, unusual among other animal behaviours (Bell et al. 2009), even after controlling for sex and territory-location factors that could inflate our estimates. Repeatability provides evidence that among-individual variation is caused by factors intrinsic to the individual (Réale et al. 2007), and the strong individual consistency in FID we have found challenges current research on this behavioural trait.
We are not attempting to raise doubts about the flexibility of animals responding to changes in predatory risk and human disturbance. The relevant question, however, is what the limits for such behavioural flexibility are. Burrowing owls breeding in territories far from roads showed larger FIDs than individuals breeding closer to roads (estimate: 2.3 ≥ 0.3, p < 0.0001, from individual model in table 1) and mated owls showed similar FIDs, which might suggest a habituation process. However, individual repeatability almost did not change when controlling for distance to roads and was much larger than repeatability within territories, there were no signs of consistent short-term habituation through repeated trials, and long-term habituation is unlikely since most individuals change mates and territories between successive years (M. Carrete & J. L. Tella 2006–2009, unpublished data). Moreover, the statistically significant reaction norm shows that, while most individuals did not change their responses, there were as many birds decreasing as increasing their FIDs across successive trials (figure 2). Remarkably, this reaction norm suggests that individuals differ in how they alter their responses to the experimental increase of human disturbance (see also Runyan & Blumstein 2004 for similar results), an intriguing result that merits further research. Thus, our study model shows that behavioural flexibility is largely constrained by an individually fixed susceptibility to disturbance. The individual consistency in FID should be tested using other species differing in life history traits and ecology.
A common strategy among studies on FID is the attempt to reduce the likelihood of resampling the same individuals. Despite not measuring individual flexibility, low FIDs have been attributed to habituation or risk-allocation processes by relating mean FID of local populations to their degree of human disturbance (e.g. Blumstein et al. 2003; Martínez-Abrain et al. 2008; Rodríguez-Prieto et al. 2009), while alternative explanations regarding inter-individual variability have not been considered. Although behavioural flexibility may allow individuals to maximize their fitness in the different environments they encounter during life, animals often show very limited behavioural plasticity and commonly differ consistently in their reaction towards similar environmental stimuli (Dall et al. 2004). Given our results, we suggest that an uneven occupation of territories by individuals with different tolerances to human disturbance may be occurring at least in some species (see also Martin & Réale 2008), so that a pattern is emerging of more tolerant individuals occupying the more disturbed environments. We expect our results will encourage others to test this phenotypic habitat-selection hypothesis.
Behavioural ecologists are increasingly finding links between individual traits (the so-called personalities, temperaments, or coping styles) and fitness (Smith & Blumstein 2008). Although more research is necessary to test whether FID is actually a temperament, for the time-being we advocate that researchers and managers take into account its individual variability, especially when developing protection zones to reduce negative effects of humans on wildlife. Otherwise, they could be favouring artificial selection of specific phenotypes at the expense of reducing behavioural diversity within a population, a diversity that may be critical to mediating responses to habitat changes and predation pressures in the long-term. Finally, given the central role of predation in the evolution of life histories, the individual consistency in FID has potential for be linked to fitness and to several other traits important to ecology and evolution, as has been suggested for some anti-predatory temperament traits (Réale et al. 2007).
Acknowledgements
We thank R. Jovani, J. Potti, F. Hiraldo, D. Serrano and two anonymous reviewers for their suggestions.
References
- Becker W. A.1984Manual of quantitative genetics Washington, DC: Academic Enterprise [Google Scholar]
- Bell A. M., Hankison S. J., Laskowski K. L.2009The repeatability of behaviour: a meta-analysis. Anim. Behav 77, 771–783 (doi:10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein D. T.2006Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim. Behav. 71, 389–399 (doi:10.1016/j.anbehav.2005.05.010) [Google Scholar]
- Blumstein D. T., Anthony L. L., Harcourt R., Ross G.2003Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol. Conserv. 110, 97–100 (doi:10.1016/S0006-3207(02)00180-5) [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 (doi:10.1111/j.1461-0248.2004.00618.x) [Google Scholar]
- del Hoyo J., Elliot A., Sargatal J.1999Handbook of the birds of the world Barcelona, Spain: Lynx [Google Scholar]
- Martin J. G. A., Réale D.2008Animal temperament and human disturbance: Implications for the response of wildlife to tourism. Behav. Proc. 77, 66–72 (doi:10.1016/j.beproc.2007.06.004) [DOI] [PubMed] [Google Scholar]
- Martínez-Abrain A., Oro D., Conesa D., Jiménez J.2008Compromise between seabird enjoyment and disturbance: the role of observed and observers. Environ. Conserv. 35, 104–108 [Google Scholar]
- McDougall P. T., Réale D., Sol D., Reader S. M.2006Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Anim. Conserv. 9, 39–48 (doi:10.1111/j.1469-1795.2005.00004.x) [Google Scholar]
- Réale D., Reader S. M., Sol D., Mcdougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 1–28 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Prieto I., Fernández-Juricic E., Martín J., Regis Y.2009Antipredator behavior in blackbirds: habituation complements risk allocation. Behav. Ecol. 20, 371–377 (doi:10.1093/beheco/arn151) [Google Scholar]
- Runyan A., Blumstein D. T.2004Do individual differences influence flight initiation distance? J. Wildl. Manag 68, 1124–1129 [Google Scholar]
- Smith B. R., Blumstein D. T.2008Fitness consequences of personality: a meta-analysis. Behav. Ecol 19, 448–455 (doi:10.1093/beheco/arm144) [Google Scholar]
- Stankowich T., Blumstein D.2005Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B 272, 2627–2634 (doi:10.1098/rspb.2005.3251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow E., Blumstein D. T.2007Evaluating methods to quantify anthropogenic stressors on animals. Appl. Anim. Behav. Sci. 102, 429–451 (doi:10.1016/j.applanim.2006.05.040) [Google Scholar]