Abstract
All animals interact with conspecifics during their life, and nearly all also display some form of aggression. An enduring challenge, however, is to understand how the experiences of an individual animal influence its later behaviours. Several studies have shown that prior winning experience increases the probability of initiating fights in later encounters. Using behavioural assays in the laboratory, we provide evidence that, in Argentine ants (Linepithema humile), the mere exposure to an opponent, without the encounter escalating to a fight, also increases the probability that it will display aggression in later encounters. Argentine ant workers differ in their propensity to attack non-colonymates, with some ants repeatedly aggressive and others consistently more docile. Although 78 per cent of the workers were consistent in their behaviour from one encounter to the next, workers that did change their behaviour after an encounter with a non-colonymate more often changed from non-aggressive to aggressive, rather than the reverse. Surprisingly, a single encounter with a non-colonymate increased a worker's propensity to fight in encounters up to a week later. An encounter with a non-colonymate also increased the probability that a worker would attack ants from a colony that it had not previously encountered. Thus, these interactions lowered the overall aggression threshold, rather than stimulating a specific aggressive response to a particular foreign colony. Finally, our data suggest that aggression towards non-colonymates increases with age.
Keywords: aggression, learning, memory, invasive species, Linepithema humile, nestmate recognition
1. Introduction
Aggression plays an important role in the acquisition and defence of resources throughout the animal kingdom. Some individuals are consistently more aggressive than others, a pattern that has been suggested to reflect individual differences in future reproductive value (Clark 1994; Wolf et al. 2007). Nevertheless, individuals may vary their behaviour based on a suite of factors, including fighting ability and resource value (Archer 1988). In addition, an individual's propensity to fight is often influenced by their prior experience (Hsu et al. 2006). Insect learning in the context of contest behaviour is well documented (Papaj & Lewis 1993; Dukas 2008). However, studies on the role of experience in aggression often focus on winning and losing experiences and as far as we know, no study has yet investigated how the mere exposure to a potential competitor influences aggressive behaviour.
In social insects, the defence of the nest and territory against foreign conspecifics is crucial for accessing and safeguarding resources. Social insect workers are able to distinguish between friend and foe with a simple sweep of the antenna across the cuticle of another individual. Previous studies of the Argentine ant (Linepithema humile) have shown that colonies become more aggressive in response to the presence of intraspecific competitors (Thomas et al. 2006, 2007). However, little is known about the mechanisms responsible for this increase in aggression. Here, we examined how the individual experiences of Argentine ant workers affect their future behaviours. In this study, we first assessed whether colonymates differ in their tendency to attack workers from different colonies. Next, we tested if the time interval between encounters influences a worker's propensity to attack non-colonymates. When levels of aggression were affected by previous encounters, we determined whether aggression was heightened specifically towards workers from the previously encountered colony, or reflect a general increase in overall aggression. Finally, we tested whether the propensity to fight changes with the age of the worker.
2. Material and Methods
(a). Nest collection and maintenance
During spring and summer 2007 and 2009, we collected colony fragments from four supercolonies in California that are genetically and behaviourally distinct from each other: Mason Park (MP), Berkeley (B), Lake Skinner (LS), Lake Hodges (LH) and Cottonwood (CW). Ants at B and MP belong to the large supercolony that dominates the introduced range in California, whereas the other three sites belong to much smaller ‘secondary’ supercolonies (Tsutsui et al. 2003; E. Van Wilgenburg 2007, personal observation). The colonies were kept in tubs lined with Fluon and fed a diet of sugar water, protein solution, scrambled eggs and crickets three times a week. Colonies were maintained in the laboratory prior to use in experiments for a maximum of four months.
(b). Behavioural assays
(i). Aggression across two successive non-colonymate encounters
To determine how consistent workers are in their aggressive behaviour, we performed a series of experiments in which we paired individual workers with non-colonymates in successive behavioural assays. To reduce ambiguities associated with reciprocal aggression, we chose pairs of colonies that were known to display a large asymmetry in aggression (Tsutsui et al. 2003) and selected workers of the more aggressive colony as our focal workers. In each assay, we marked the focal worker by applying non-toxic acrylic paint to its abdomen. We allowed the worker to recover from handling for approximately 5 min. Next, we introduced a worker from a foreign colony (contestant no. 1) and observed worker behaviour in a 35 mm, Fluon-coated Petri dish for a maximum of 3 min. We scored the assay as aggressive if the marked worker showed one or more of the following behaviours: flaring of mandibles, recoil behaviour, biting or grabbing. After either 3 min or the first incidence of aggression (by either ant), we removed the workers from the Petri dish. Then, 15 min later, we paired the focal worker in a second behavioural assay, following the same format as the first, but with a different individual (contestant no. 2) from the same colony as contestant no. 1. We performed at least 50 replicates per treatment, for four colony combinations (LH as focal ants against CW, LH–LS, B–CW and MP–CW). One replicate is one focal worker used in a series of two assays.
(ii). Colony specificity in aggression
To investigate the colony specificity of worker aggression, we performed the same assay as above, but tested each focal ant against two non-colonymates from two different colonies. We performed at least 50 replicates per treatment, for two colony combinations (LH–LS then against CW; LH–CW then LS).
(iii). Aggression over time
To determine if the amount of time between aggressive encounters affects whether worker aggression remains consistent, we performed the same procedure as in the first experiment above, but included three different time intervals (1 h, 1 day or one week) between the first and second assays. For the 1 day and one week intervals, we returned the focal workers to small laboratory colonies (approx. 200 workers and two queens) after the first behavioural assay. To keep track of the aggressive and non-aggressive workers from the first assays, we put the aggressive workers in a separate laboratory colony from the non-aggressive workers. We performed at least 50 replicates per treatment, for three time intervals, for three colony combinations (1 h: LH–CW, LH–LS, MP–CW, B–CW; 1 day: MP–CW, B–CW; one week: MP–CW). We performed controls by placing a focal worker in a Petri dish for 3 min before pairing it with a contestant 15 min, 1 h or 1 day later (B against CW, 50 replicates per time interval). To investigate how worker aggression changes after more than two intercolony encounters, we performed behavioural assays that paired individual workers with a series of 10 different non-colonymates at 15 min intervals (LH against LS, MP against CW, 50 repeats each).
(iv). Worker age and propensity to fight
To determine whether aggression increases with worker age, we tested the behaviour of workers of known age towards non-colonymates. We constructed two small laboratory colonies (from MP), each consisting of two queens, approximately 1000 workers and 100 pieces of brood. To track the age of workers, we added cohorts of between 50 and 200 newly eclosed workers, each marked with a unique colour of acrylic paint, to each tub at five different times. When the first behavioural assays were performed, the laboratory colonies contained marked cohorts of five approximate ages: eight, six, four, one week and 1 day. On the day of testing, we removed all the painted workers and tested whether they were aggressive towards workers from a foreign colony (CW). After we completed the assays we returned the painted workers to their original colony fragment. We conducted a second round of assays two or four months after the first assay.
(c). Statistical analysis
Analyses were carried out using Genstat (v. 6). We used generalized linear mixed models (GLMM) or restricted maximum likelihood models (REML) with colony identity of the painted worker as a random factor, to accommodate repeated measures of each colony.
3. Results
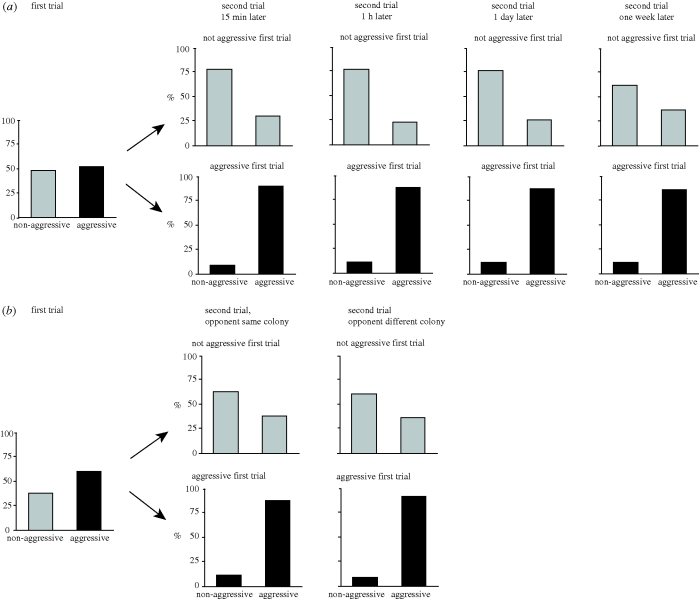
We found that 78 per cent of Argentine workers were consistent in their behaviour (either aggressive or not aggressive in both assays) when presented with non-colonymates in two successive assays. The probability of aggression in the second assay was significantly higher for workers that were aggressive in the first assay than for those that were not (figure 1; 89% versus 29%, χ2 = 197.51, p < 0.001), and this was not significantly influenced by the time interval between assays (15 min, 1 h, 1 day or one week) (figure 1, χ2 = 3.84, p = 0.280) or whether the non-colony mates in the first and second assay were from the same or from different colonies (figure 1, χ2 = 0.42, p = 0.518). For those workers that did exhibit a behavioural change, it was more common for workers to switch from non-aggressive to aggressive (68% of workers that switched) than to switch from aggressive to non-aggressive (figure 1; 2% of workers that switched). This difference resulted in a significant overall increase in the occurrence of aggression between the first and the second assays (52% and 63%, respectively, a 21% increase (χ2 = 13.37, p < 0.001)). We found that the increase in aggression between the first and second assays was not significantly influenced by the time interval between assays (figure 1a; χ2 = 0.05, p = 0.997) or whether the opponents in the first and second assay were from the same or different colonies (figure 1b; χ2 = 0.01, p = 0.912). Although aggression was usually initiated by the focal worker, in 7 per cent of the assays, aggression was initiated by the non-colonymate being presented to the focal ant. Being attacked by the non-focal worker in the first assay did not significantly influence the probability that the focal worker would be aggressive in the second assay (χ2 = 0.26, p = 0.610). Workers that were aggressive in both assays did not attack more quickly in the second assay than they did in the first (REML; χ2 = 2.33, p = 0.127).
Figure 1.
Consistency in aggression across two successive encounters (a) separated by various amounts of time or (b) across encounters with non-colonymates of two different colonies. The panels on the left shows the initial proportion of aggressive (black bars) versus non-aggressive interactions (grey bars). These focal workers were used in a second trial at either 15 min, 1 h, 1 day or one week after the first assay (top right panels) or in a second trial 15 min after the first assay against contestants of the same or a different colony (bottom right panels).
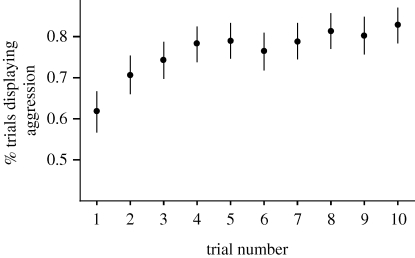
Overall, workers that had been exposed to a non-colonymate were always more aggressive towards non-colonymates in the second assay than ants of the control treatments (χ2 = 13.56, p < 0.001) regardless of time spent in Petri dishes (15 min or 1 h) or in a small laboratory colony (1 day) (χ2 = 0.93, p = 0.63). Moreover, the average frequency of aggression displayed by individuals continued to increase over 10 successive encounters with non-colonymates (figure 2; χ2 = 10.75, p = 0.001).
Figure 2.
Percentage of trials (±s.e.) in which workers displayed aggression towards conspecific workers during 10 successive assays.
The propensity of workers to fight increased significantly with worker age (GLMM estimated effect = 0.01±0.004, χ2 = 5.17, p = 0.023). This increase in aggression did not appear to occur after the age of three months. However, since the number of workers we were able to retrieve declined with worker age, our sample sizes of workers older than three months were very small, reducing the power for analysis of these age classes.
4. Discussion
Here we show that there is substantial variation in aggressive behaviour between individuals from within the same colony and that this aggressive behaviour can increase with experience. Theoretical studies on non-social animals suggest that differences in risk-taking behaviour between individuals can be a consequence of differences in reproductive value (Clark 1994; Wolf et al. 2007). However, social insect workers are often sterile, so their death results in a loss of workforce, rather than a loss of opportunity to reproduce. Instead, the use of specialized individuals to perform intercolonial aggression may benefit the colony overall. For example, colonies may conserve resources by allocating older workers of the colony to fighting. Accordingly, we found that older workers were more likely than younger workers to attack non-colonymates.
Previous studies of the Argentine ant have shown that colonies become more aggressive in response to the presence of intraspecific competitors (Thomas et al. 2006, 2007). Here we show how this operates at the level of individual workers: for some workers, multiple encounters with a foreign individual are necessary to trigger aggression. Probably this increase in aggression is not owing to associative learning by the focal workers, but rather to sensitization, since being attacked by the non-focal worker in the first assay did not significantly influence the probability that the focal worker would be aggressive in the second assay. Such learning effects on task performance, or changes in response thresholds, have been demonstrated in ants in other contexts, such as nest relocation (Langridge et al. 2008). However, to our knowledge we are the first to report how experience alters aggressive behaviour in ants.
Learning in the context of aggression has been demonstrated in a variety of other taxa. In particular, it has been shown that prior winning experience can increase the probability of initiating fights in later encounters (Hsu et al. 2006). For example, when paired with similar sized opponents, male spiders (Argyrodes antipodiana) with previous winning experience were more likely to win against spiders that had previously lost a contest (Whitehouse 1997). Our results differ in that we show that the mere exposure of a worker to an opponent, without the encounter escalating to a fight, also increases the probability it will display aggression in later encounters. Moreover, we show that increased worker aggression towards foreign workers is not colony-specific. Thus, exposure to a foreign conspecific appears to lower the threshold for aggression rather than causing workers to learn to recognize the specific odour of a foreign colony.
Acknowledgements
We would like to thank M. A. Smith, C. Huang, M. Ruderman and D. Camarilla for assisting with the behavioural trials and P. Lester and an anonymous referee for useful comments on the manuscript. Funding for this work was provided by the Defining Wisdom programme (a project of the Arete Initiative at the University of Chicago; N.D.T), the US Department of Agriculture (NRI-CGP 2004-3502-14865; to N.D.T) and the California Structural Pest Control Board (N.D.T).
References
- Archer J.1988The behavioural biology of aggression New York, NY: Cambridge University Press [Google Scholar]
- Clark C. W.1994Antipredator behaviour and the asset-protection principle. Behav. Ecol. 5, 159–170 (doi:10.1093/beheco/5.2.159) [Google Scholar]
- Dukas R.2008Evolutionary biology of insect learning. Ann. Rev. Entomol. 53, 145–160 (doi:10.1146/annurev.ento.53.103106.093343) [DOI] [PubMed] [Google Scholar]
- Hsu Y. Y., Earley R. L., Wolf L. L.2006Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33–74 (doi:10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- Langridge E. A., Sendova-Franks A. B., Franks N. R.2008How experienced individuals contribute to an improvement in collective performance in ants. Behav. Ecol. Sociobiol. 62, 447–456 (doi:10.1007/s00265-007-0472-5) [Google Scholar]
- Papaj D. R., Lewis A. C.1993Insect learning New York, NY: Chapman & Hall [Google Scholar]
- Thomas M. L., Payne-Makrisa C. M., Suarez A. V., Holway D. A.2006When supercolonies collide: territorial aggression in an invasive and unicolonial social insect. Mol. Ecol. 15, 4303–4315 (doi:10.1111/j.1365-294X.2006.03038.x) [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Payne-Makrisa C. M., Suarez A. V., Tsutsui N. D., Holway D. A.2007Contact between supercolonies elevates aggression in Argentine ants. Insect. Soc. 54, 225–233 (doi:0.1007/s00040-007-0935-8) [Google Scholar]
- Tsutsui N. D., Suarez A. V., Grosberg R. K.2003Genetic diversity, asymmetrical aggression, and recognition in a widespread invasive species. Proc. Natl Acad. Sci. 100, 1078–1083 (doi:10.1073/pnas.0234412100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse M. E. A.1997Experience influences male–male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae). Anim. Behav. 53, 913–923 (doi:10.1006/anbe.1996.0313) [Google Scholar]
- Wolf M., Van Doorn G. S., Leimar O., Weissing F. J.2007Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]