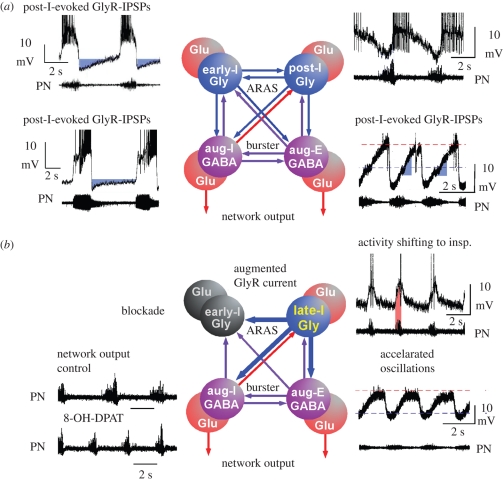
Figure 6.
Proposal of the network reorganization following global stimulation of 5-HT1AR. Based on the experimental data obtained in mouse, rat and cat, we propose a functional reorganization of network operation. (a) Starting from control, we conclude that the respiratory network reveals a mutual inhibitory organization between antagonistic groups of neurons operating either via GABAergic synaptic interaction (violet cells and arrows) or through glycinergic interactions (blue cells and arrows (ARAS, ascending reticular activating system; IPSPs, inhibitory postsynaptic potentials)). Activation of both inhibitory processes is fully controlled by excitatory glutamatergic neurons (red cells) that determine the neural output to respiratory and laryngeal muscles through polysynaptic bulbar and bulbo-spinal connections. GABAergic and glycinergic neurons form the indispensable structure for mutual inhibition. There is a reciprocal GABAergic synaptic interaction between inspiratory (aug-I) and expiratory (aug-E) neurons generating an alternating pattern of augmenting activity that is necessary for breathing movements. Equally important seem to be the inhibitory interactions between early-inspiratory (early-I) and post-inspiratory (post-I) neurons that control phase transitions between respiratory cycles. In the original recordings (from cat) depicted beside each neuron population, hyperpolarizing voltage trajectories generated by glycinergic inhibition of the different types of neurons are labelled in blue. (b) 5-HT1AR-mediated signalling induces a functional reorganization of network operation that originates from a potentiation of glycine receptors (GlyR) as illustrated by thick arrows indicating synaptic interconnections. The firing of post-I neurons shifts into the inspiratory phase (inset of original recording, pink area), which seems to powerfully suppress early-I neuronal discharges by enhanced GlyR currents. Suppression of early-I neurons is evidenced by the disappearance of inspiratory inhibition of post-I neurons. As post-I neurons fire prematurely during inspiration, enhanced glycinergic inhibition of aug-I neurons effectively shortens inspiratory burst duration, but increases burst frequency of inspiratory neurons as seen in the inspiratory output in the phrenic nerves (PN).