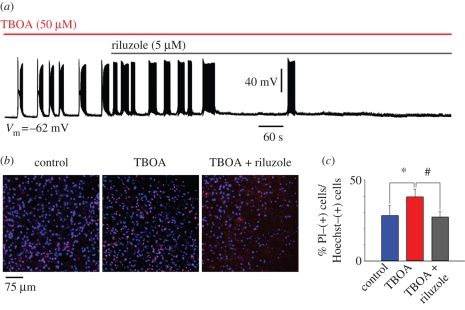
Figure 3.
Riluzole inhibits TBOA-evoked bursting. (a) Sample trace of current clamp recording from hypoglossal motoneuron exposed to TBOA (50 µM) that, on average, produces 3.0 ± 0.7 mV depolarization (n = 8; data not shown). In this example (recorded in the presence of 0.4 µM strychnine and 10 µM bicuculline), bursts emerge with a latency of 60 s. Note that the burst structure is made up of a depolarization envelope with superimposed fast spikes followed by transient inactivation and subsequent return of firing. This pattern confers a ‘butterfly’ shape to each burst event. Riluzole (5 µM) added after 4.30 min from the start of TBOA application slowly inhibits bursting, which, in this example, is suppressed after 10 min. (b) Histochemical demonstration of motoneuron damage produced by 60 min application of TBOA and its antagonism by riluzole (applied 14 min from the start of TBOA application). Cell damage is assessed with propidium iodide (a DNA dye that labels membrane-damaged cells; Sharifullina & Nistri 2006) staining (shown in red) versus staining due to Hoechst 33342 (shown in blue; a cell-permeable DNA dye). (c) Histograms quantify increased death of motoneurons after exposure to TBOA (n = 13) and its prevention by riluzole (n = 23), which preserves the same number of surviving cells found in control conditions (n = 19). Data are expressed as percentage of propidium iodide-positive cells versus Hoechst 33342-positive cells and quantified with ImageJ software. *p = 0.038 for the difference between control and TBOA, and #p = 0.025 for the difference between TBOA and TBOA plus riluzole. Slices were collected from 10 rats (A. Cifra 2008, unpublished data).