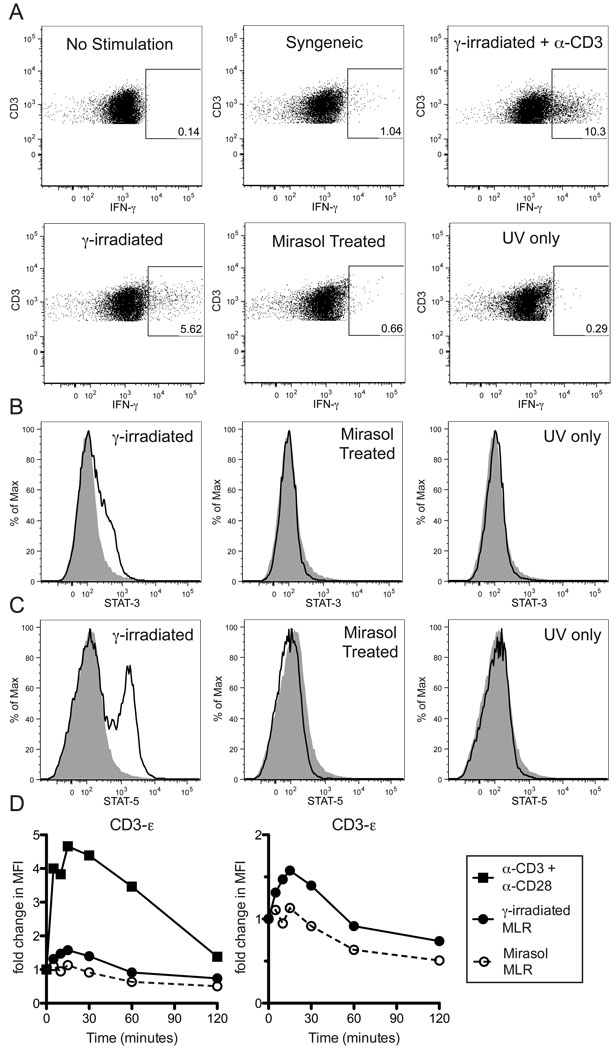
Figure 6. Mirasol treated are cells unable to activate untreated T cells.
Normal PBMCs were primed with γ-irradiated cells to enrich for allo-specific cells. These primed cells were labeled with CFSE, then restimulated overnight with γ-irradiated, Mirasol treated, or UV only treated allogeneic cells from the same donor as the priming cells. Additional groups that were either unstimulated, or stimulated with syngeneic cells or γ-irradiated allogeneic cells + α-CD3 antibodies were included as controls. CFSE+CD3+ gates were used to identify responder T cells (A). Gates are drawn around IFN-γ+ T cells based on unstimulated control and confirmed with isotype control stains. A minimum of 8,000 T cells were collected for each sample. This experiment was repeated three times with representative data displayed. In B–C the primed cells were restimulated with labeled cells (from the same donor as the priming cells) that were either γ-irradiated, Mirasol treated, or UV only treated for two hours, then stained intracellularly for STAT-3, STAT-5, and CD3. Stains of responder T cells for STAT-3 (B) and STAT-5 (C) are plotted with the unstimulated control stain shown in grey for comparison. In D, normal PBMCs were stimulated with anti-CD3 + anti-CD28 antibodies ■, and primed cells were restimulated with γ-irradiated cells ●, or Mirasol treated cells ○ (from the same donor as the priming cells), for 0, 5, 10, 15, 30, 60, or 120 minutes, lysed, and examined for the phosphorylation of CD3-ε using a multiplex bead-based kit. Fold change over t=0 is plotted over time. The left panel shows all three stimulation conditions, the right panel shows only the two MLR stimulations. Experiments in B–D were repeated twice with representative data displayed.