Abstract
Vector-mediated cardiac gene therapy holds tremendous promise as a translatable platform technology for treating many cardiovascular diseases. The ideal technique is one that is efficient and practical, allowing for global cardiac gene expression, while minimizing collateral expression in other organs. Here we survey the available in vivo vector-mediated cardiac gene delivery methods—including transcutaneous, intravascular, intramuscular, and cardiopulmonary bypass techniques—with consideration of the relative merits and deficiencies of each. Review of available techniques suggests that an optimal method for vector-mediated gene delivery to the large animal myocardium would ideally employ retrograde and/or anterograde transcoronary gene delivery,extended vector residence time in the coronary circulation, an increased myocardial transcapillary gradient using physical methods, increased endothelial permeability with pharmacological agents, minimal collateral gene expression by isolation of the cardiac circulation from the systemic, and have low immunogenicity.
Introduction
One prerequisite for effective cardiac gene therapy is the need for a reliable, safe, and clinically relevant delivery system to the human myocardium. Although a great number of methods have been identified, each has significant limitations. The theoretically ideal method of cardiac gene delivery would be to use a vector that could be administered intravenously, with globally efficient and specific uptake into cardiac myocytes (Vinge et al., 2008). Unfortunately, in spite of the development of adeno-associated viral (AAV) vector serotypes with significant tropism for the heart and in spite of the extremely promising findings in murine species (Gregorevic et al., 2004; Wang et al., 2005; Inagaki et al, 2006), these results have not yet been confirmed using an intravenous delivery route in small or large animals in situ. Furthermore, on the basis of previous murine studies (Gregorevic et al., 2004; Wang et al., 2005; Inagaki et al., 2006), the doses of vector required to transduce the human heart by intravenous injection would likely be prohibitive or certainly impractical. Thus, the quest for more efficient, cardiac gene delivery methods is currently a critically important, rate-limiting challenge in clinical cardiac gene therapy.
The intent of this review is to provide a systematic classification of available in vivo gene delivery techniques, to elucidate the relative merits and deficiencies of the various methods, and to highlight the potential for incorporating effective gene therapy into the therapeutic armamentarium for clinical management of cardiovascular disease. We focus on methods of vector-mediated gene delivery applicable to the treatment of heart failure, keeping in mind that there are still many inherent issues associated with this technology platform that remain unresolved. Prevailing questions include the following: what are the appropriate levels of gene transcription and translation within the transfected cardiomyocytes of the failing heart (Lyon et al., 2008); does restoration of myocardial contractility require gene transfer to the majority of target cells (Ly et al., 2007); which vector serotypes have the greatest cardiac myocyte tropism with minimal untoward systemic effects (Kizana and Alexander, 2003; Bish et al., 2008); what fraction of cardiac myocytes need to be transfected to obtain global gene delivery; is desirable long-term and homogeneous gene expression by the majority of cardiac myocytes a requirement or is regional gene expression sufficient?
We selected the following general criteria to assess the efficacy of a given delivery method:
Distribution of myocardial transduction (global vs. regional)
Efficiency of myocyte transduction (percentage of myocytes transduced/multiplicity of infection)
Technical difficulty, complications, and physiological effects associated with use of the proposed method
Delivery-related specificity of transgene expression to the myocardium: evidence of collateral transgene expression in distant organs (independent of promoter tissue specificity)
Delivery-related inflammatory response
We then further classify the available methods of cardiac gene delivery on the basis of the following criteria:
-
Location of vector injection
Intramyocardial
Intrapericardial and epicardial
Endocardial
-
Intravascular
Intravenous (systemic, retrograde coronary; e.g., coronary sinus)
Intraarterial (anterograde coronary)
Intracavitary, intraventricular, intraatrial
-
Surgical/interventional approach
Percutaneous
Thoracoscopic
Minimally invasive
Thoracotomy
Sternotomy
-
Method of cardiac perfusion during gene delivery
Beating heart (native circulation)
Ex vivo perfusion (e.g., before transplantation)
Cardiopulmonary bypass in the arrested heart
We focus on methods of vector-mediated gene delivery applicable to the treatment of heart failure. The intent was to present these techniques in historical chronology or in the order of relevance as new developments contributed to refinement of cardiac gene delivery methodologies. In addition, we separately address anterograde and retrograde coronary gene delivery, as varying authors using these methods have demonstrated opposing results.
Intramyocardial Injection
Several laboratories reported, almost simultaneously, the expression of naked recombinant DNA injected into the murine heart and later in larger mammals. Lin and colleagues (1990) demonstrated that the lacZ gene could be introduced and expressed in cardiac myocytes after direct injection of DNA into the left ventricular wall via a left thoracotomy. Expression, however, was patchy and was observed only within a few millimeters of the injection site (Buttrick et al., 1992; French et al., 1994). The absolute amount of recombinant protein produced by plasmid injection was small and given the limited distribution of transduced myocytes, alternatives were considered. The first use of replication-deficient adenovirus for intramyocardial injection into a large animal (porcine) species resulted in a 140,000-fold increase in the ratio of recombinant protein produced to the number of genomes injected compared with the injection of plasmid DNA. However, difficulties with delivery were still significant, with little or no gene expression 5 mm from the injection site and transient gene expression, declining progressively after 14 days. Furthermore, adenoviral transfection resulted in an aggressive cell-mediated immune response (French et al., 1994).
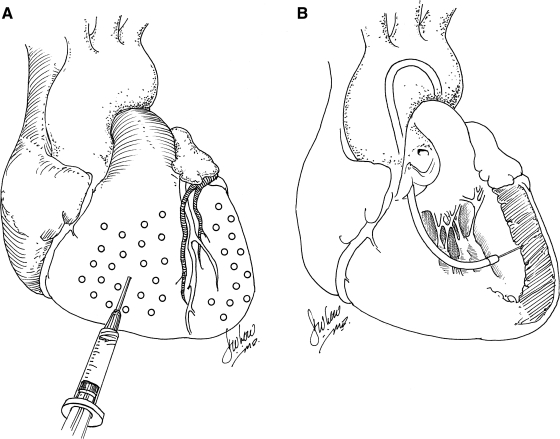
Although early studies of gene transfer using intramyocardial injection of plasmid DNA or adenovirus expressing reporter genes were encouraging, estimates of the number of myocytes that could be transfected in vivo were low, primarily as a result of the inability to achieve a uniform distribution of vector delivery more than a few millimeters from each injection site. These concerns—which persist to the present time—continue to make clinically relevant therapeutic efficacy unlikely, using this approach. A potential solution to the regional, rather than global, transduction associated with intramyocardial injection is to attempt multiple injections spatially and temporally. Multiple injections over a broad distribution (spacing of 1 cm), for example, in order to achieve gene expression over a clinically relevant territory of myocardium, was performed by French and colleagues (1994), using a 4 × 4 cm grid to define the sites of injection and to avoid coronary vessels (Fig. 1A). In spite of the relatively close spacing of injection sites, extremely inhomogeneous gene transfer was achieved in a porcine model (French et al., 1994), using adenovirus encoding LacZ. Using a rat model, Guzman and colleagues (1993) confirmed that adenovirus was several orders of magnitude more efficient in transducing cardiac myocytes after intramyocardial injection than plasmid DNA expressing the same construct. For plasmid DNA, it was also demonstrated that expression of injected gene constructs is dose dependent and has kinetic features of a saturation curve at doses exceeding 200 μg per injection site. Increasing the amount of DNA to 300 μg did not result in an increase in recombinant protein production. This may have resulted from saturation of the available sites for DNA uptake, although the mechanism remains unknown (von Harsdorf et al., 1993). In this regard, using a porcine model, Grossman and colleagues (2002) found that at endocardial injection volumes of 10 μl, most injected microspheres are retained in the myocardium; whereas at injection volumes of 100 μl (commonly used in clinical trials), only 20% are retained and only 10% are retained when administered via epicardial injection. Therefore, above a certain threshold, higher doses of vector administered intramyocardially may result in a plateau in myocardial gene expression but an increase in collateral gene expression. Interestingly, in a report by Bish and colleagues (2008), using a canine model with a 250-μl injection volume per site, the concentration ratio of injected vector genomes, using adeno-associated viral vectors, in the liver to the heart (liver genome copies [gc]/ml)/(cardiac gc/ml) was greater than 1 for two of the four AAV serotypes tested. Considering that the liver mass averages four times the cardiac mass, these results show that intramyocardial injection often results in the majority of vector delivery to organs other than the heart, due to significant spillage of vector constructs into the systemic circulation. Thus, intramyocardial injection does not typically result in tissue-specific gene expression when a constitutive promoter is used.
FIG. 1.
(A) Intramyocardial gene delivery with syringe. (B) Catheter-mediated percutaneous endomyocardial gene delivery.
Another unresolved issue in using direct intramyocardial injection involves an acute inflammatory response. Studies suggest that a specific component of the inflammatory response is likely secondary to injury produced by direct injection rather than by an evoked immunologic response to viral gene constructs (Guzman et al., 1993). In support of this view, it has been further noted that this response is not dose dependent (von Harsdorf et al., 1993). Although not dependent on the route of delivery, the transfection of cardiomyocytes with high doses of adenovirus serotype 5 (Ad5) vector gene constructs was associated with marked leukocytic infiltration, which presumably limited the intensity and duration of recombinant gene expression and caused significant tissue damage (French et al., 1994). Delivery of adenoviral constructs by intravenous delivery routes also results in a significant inflammatory response, but evidence from studies in skeletal muscle indicates that the inflammatory response after intramuscular injection is considerably more robust than the response to vector delivered via the transvascular route (Ohshima et al., 2009).
In summary, intramyocardial gene delivery studies have demonstrated that direct, in vivo gene transfer into ventricular cardiac myocytes is possible using both naked DNA and viral vectors. Gene expression does not appear to be limited exclusively to cardiac muscle, in spite of the local, rather than systemic, method of gene delivery and significant transgene expression may occur in other organs. Saturation kinetics are likely relevant because, as the dose and volume of injectate increase, increased spillage of vector into the systemic circulation becomes likely, resulting in a fixed upper limit of gene expression in the heart with increasing levels of expression in other organs.
The major advantage of direct intramyocardial injection is its simplicity and safety, as it is possible to provide injection percutaneously (Bish et al., 2008) or with a minimally invasive surgical thoracotomy incision. Clinical trials using this technique have been performed (Losordo et al., 1998; Rosengart et al., 1999). Unfortunately, these phase I studies have not measured the amount of recombinant protein produced; hence, it has not been possible to make accurate, quantitative determinations of the relative efficiency of vector-mediated gene transfer, nor has a therapeutic effect been demonstrated. Despite its inhomogeneous distribution of transgene delivery, this methodology has resulted in therapeutic efficacy in some experimental models. For example, it has been shown that it is possible to augment cardiac function in cardiomyopathic hamsters by injecting plasmid constructs encoding the β2-adrenergic receptor (β2-AR) gene directly into the myocardium of cardiomyopathic hamsters (Tomiyasu et al., 2000). However, given the nonscalability of the intramuscular delivery method, promising results in murine models are unlikely to translate directly to larger mammals such as humans.
Intrapericardial Gene Transfer
Lamping and colleagues (1997) hypothesized that increasing the duration of exposure of adenoviral vector in the pericardial space would result in gene expression in the pericardium and probably the myocardium. In these studies, expression predominated in the parietal pericardium. Those data were confirmed by March and colleagues (1999), who described a technique of percutaneously introducing a helical penetrating catheter into the canine right ventricular intrapericardial space. Fromes and colleagues (1999) showed that injecting a mixture of proteolytic enzymes, together with an adenovirus carrying the lacZ gene, into the murine pericardial sac leads to diffusion of the gene construct with expression in the left ventricle and the interventricular septum. Zhang and colleagues (1999) also found that intrapericardial injection of adenoviral vectors in murine neonates resulted in transmural expression of reporter genes, but the volume of injectate was a major determinant of the transduction efficiency. In addition, there was a high level of hepatic transduction and no persistence of LacZ expression in the ventricles. It is important to note that high levels of systemic gene transfer were also obtained after intrapericardial injection of viral vectors in murine neonates, an observation that is not translated to adult mice. Thus, the intrapericardial route is not likely to be particularly promising for clinical application in adult patients.
Endocardial Gene Transfer
Percutaneous myocardial gene transfer was achieved in normal and ischemic myocardium after endocardial delivery using an electromechanical mapping system for three-dimensional image reconstruction of the ventricles in a study by Vale and colleagues (1999). In porcine subjects, an injection catheter was used to deliver plasmid DNA encoding a cytomegalovirus (CMV) promoter-driven lacZ gene to a single region of the left ventricle. Significant peak β-galactosidase activity was evident after 5 days in the target area. Later studies illustrated the feasibility of electromagnetic (Kornowski et al., 2000; Sylvén et al., 2002) and fluoroscopic guidance (Gwon et al., 2001; Sanborn et al., 2001; Bish et al., 2008) for catheter-based, transendocardial injection. In one study, 15 porcine subjects underwent myocardial injection using a percutaneous endomyocardial catheter (Fig. 1B). Multiple neutron-activated microsphere species were used as tracers. Animals were killed immediately and microsphere species were quantified. It was found that endomyocardial injection was associated with significantly higher microsphere retention than open chest epicardial injection (Grossman et al., 2002). These results notwithstanding, one would expect, a priori, that all of the limitations of epicardial injection—saturation kinetics, collateral gene expression, inhomogeneous distribution, and immunogenicity—would apply to endocardial injection as well.
Insights Gained from Langendorff Perfusion Model
Experiments involving Langendorff perfusion, a model in which the aorta is cannulated and the heart is perfused in a retrograde fashion with reservoirs that provide pressure and flow, led to better understanding of the factors affecting gene transfer by intracoronary delivery of adenovirus to intact leporine hearts. These studies illustrated the need to monitor the coronary flow rate, the absolute amount of virus, the contact time with the coronary circulation, and the composition of the perfusate (Donahue et al., 1997, 1998). It was demonstrated that there is a direct link between increasing the coronary flow and transfection efficiency and further suggested that manipulations that improve gene transfer are likely mediated by facilitated opening of precapillary sphincters, allowing free movement of the recombinant adenovirus into the interstitium. The presence of heparinized, leporine blood caused a 60% reduction in the number of transfected cells compared with crystalloid solution alone. This observation was attributed to the nonspecific adherence of viral particles to red blood cells and lower perfusate calcium concentration. It was additionally found that the incorporation of endothelial permeabilizing agents improved transfection efficiency. One highly relevant finding was that a single pass of the virus solution through the heart caused transduction of only 0.8% myocytes in contrast to 40% when the virus-containing perfusate was recirculated for 60 min (Donahue et al., 1997).
Cardiopulmonary Bypass
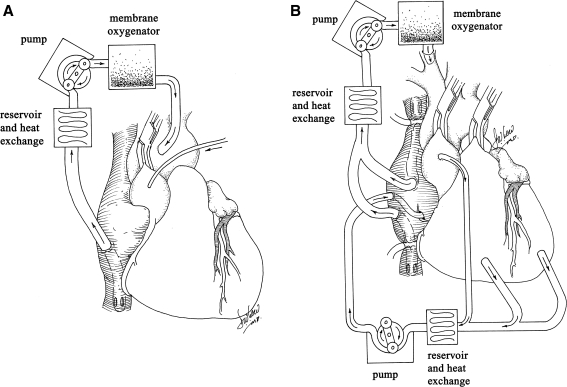
Davidson and colleagues (2001) and Bridges and colleagues (2002) first hypothesized that cardiopulmonary bypass (CPB) may facilitate cardiac-selective gene transfer using recombinant replication-deficient adenovirus. CPB is the method whereby the cardiac and pulmonary circulations are bypassed, using a heart–lung machine to simulate the critical functions of the heart and lungs: retrieval of deoxygenated blood from the systemic venous circulation and pumping oxygenated blood, after removal of carbon dioxide, to the systemic arterial circulation. The rationale for using CPB for cardiac gene therapy is that the cardiac (coronary) circulation can be separated from the systemic circulation, blood cells can be removed from the coronary circulation, and the temperature of the perfusate can be manipulated to potentially enhance gene transfer. Davidson and colleagues (2001) demonstrated the feasibility of myocardial gene delivery during CPB with cold, hyperkalemic cardioplegic arrest in the porcine model (Fig. 2A). The absence of a significant influence of cold temperatures on transgene expression in an in vivo model with CPB was described by Jones and colleagues (2002). This work also demonstrated that using aortic root injection, transgene expression in the right ventricle was considerably less than in the left ventricle and that the presence of crystalloid cardioplegia (myocardial protection solution), compared with blood cardioplegia within the coronary circulation, had no effect on transgene expression. It was hypothesized that endothelial contact with cardioplegia and the associated relative ischemia likely increased endothelial permeability, thus facilitating vector-mediated transfection (Jones and Koch, 2005). Ikeda and colleagues (2002) evaluated the method of vector delivery using cardiac arrest with deep and mild hypothermia in cardiomyopathic hamsters, followed by the delivery of cold crystalloid cardioplegia containing histamine. This approach produced a marked increase in transfection efficiency with homogeneous β-galactosidase staining of most cardiac myocytes versus the conventional aortic root injection group, and there was no significant difference between experiments with mild and deep hypothermia.
FIG. 2.
(A) Gene delivery via conventional cardiopulmonary bypass with cardioplegic arrest. (B) Gene delivery via cardiopulmonary bypass with cardioplegic arrest, using molecular cardiac surgery with recirculating delivery (MCARD) with complete cardiac isolation and “closed loop” recirculation.
Unlike Davidson and colleagues (2001), who used a single-pass perfusion technique, Bridges and colleagues (2002) were the first to create an isolated “closed-loop” recirculating model of vector-mediated cardiac gene delivery in the large animal heart, using cardiopulmonary bypass with an anterograde delivery approach allowing for vector recirculation for 20 to 30 min (Fig. 2B). Using separate CPB circuits for the cardiac and systemic circulations, this system allows for the physical separation of arterial inflow and venous effluent from the two circuits, thus making it possible to achieve complete cardiac isolation, simultaneously increasing the concentration and contact time of the administered vector in the coronary circulation and minimizing collateral organ gene expression because the vector is cleared from the cardiac circulation before weaning from CPB (Bridges et al., 2002). Although transfection efficiency and cardiac specificity were improved by this technique, this group subsequently demonstrated that complete surgical isolation of the heart in situ, using CPB with high-pressure retrograde coronary sinus infusion with multiple-pass recirculation of vector through the heart, results in an increase of several orders in the magnitude in β-galactosidase activities in the heart compared with controls that received retrograde infusion of adenovirus without CPB and without cardiac isolation (Bridges et al., 2005). This closed-loop method allows for multiple pass cardiac recirculation, increased contact time with the coronary circulation, control of temperature and ionic composition of the perfusate, removal of blood cells, and addition of endothelial permeabilizing agents, each of which has been shown to increase transfection efficiency. This methodology, referred to as “molecular cardiac surgery with recirculating delivery” (MCARD), has been used to deliver self-complementary AAV6, encoding both EGFP and βARKct to the ovine myocardium, resulting in robust global cardiac-specific gene expression (Swain et al., 2009; White et al., 2009a,b). The major limitation of the MCARD technique, however, is that CPB is required with its attendant potential morbidity. This limitation may be mitigated by the fact that some of the patients for whom the technique may be ultimately intended would be undergoing CPB for other reasons.
Gene Transfer Using Retrograde Coronary Delivery
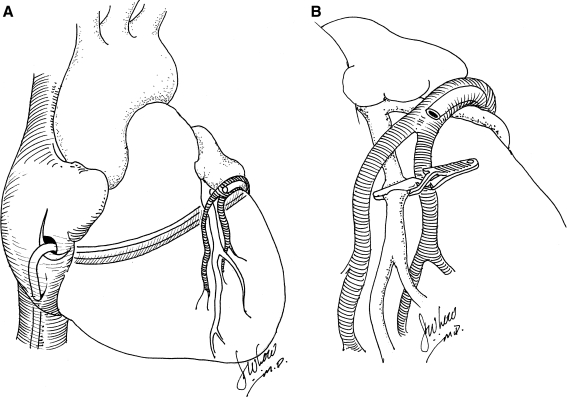
Boekstegers and colleagues (2000) studied the effect of retrograde gene delivery through the coronary veins on the beating porcine heart. The authors used a self-engineered, technical-assist device that consists of a pumping unit for arterial blood withdrawal, a small extracorporeal circuit, a retroinfusion catheter, and a suction device. This group found that selective pressure-regulated retroinfusion of the coronary veins prolongs adhesion time of the vector with the cardiac endothelium and increases endothelial permeability. In contrast to anterograde intracoronary delivery, retroinfusion was able to increase adenoviral gene transfer to the targeted myocardium (Fig. 3A). In the ischemic myocardium, because of the venous delivery route, gene expression was distributed more homogeneously. Furthermore, overall gene expression in the targeted left anterior descending artery (LAD) region after adenoviral gene transfer was superior to that achieved after intramyocardial injection (Boekstegers and Kupatt, 2004; Raake et al., 2004) (Fig. 3B).
FIG. 3.
(A) Catheter-medicated percutaneous retrograde gene delivery through the coronary venous system in the beating heart. (B) Catheter-mediated percutaneous retrograde gene delivery though the coronary venous system with concomitant myocardial ischemia in the beating heart.
Using a slightly different approach, Hou and colleagues (2003) studied retrograde coronary venous myocardial gene delivery by the percutaneous approach on the porcine beating heart. Single retrograde administration using a balloon-inflated catheter of a plasmid encoding human Del-1 resulted in efficient regional myocyte transfection. The authors explained that the coronary venous approach offers direct local delivery into the interstitium of the myocardium with minimal washout and allows for controlled dwell times for longer exposure. This view was confirmed in studies by Bridges and colleagues (2005) and Su and colleagues (2005), which have also shown that a retrograde infusion approach both in the isolated limb and in the heart results in enhanced transduction efficiency. The rationale for this increased transfection efficiency of the retrograde venous-to-arterial route is that retrograde vector infusion results in a higher pressure gradient for filtration because both venular and capillary filtration occur on the venous side of the arteriolar resistor (Hou et al., 2003; Bridges et al., 2005; Su et al., 2005).
Anterograde Intracoronary Viral Gene Delivery
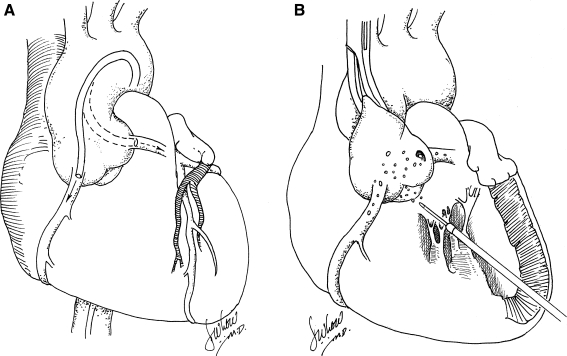
Creation of an efficient and homogeneous method for gene delivery during percutaneous coronary intervention would be beneficial, especially if gene therapy for heart failure is to be applied to humans (Hayase et al., 2005). The feasibility of in vivo cardiac gene transfer with the aid of percutaneous catheter-mediated intracoronary delivery has been demonstrated in many studies, and some authors believe that it is the most clinically relevant method because of the possibility of delivering vectors to the whole myocardium and because of the extensive clinical experience in coronary catheterization procedures (Shah et al., 2000a,b; Logeart et al., 2001) (Fig. 4A). However, the efficiency of adenovirus and adeno-associated virus-mediated gene delivery by this method is highly variable among studies. Simple intracoronary catheterization with in vivo models succeeded in broadening the distribution of adenovirus delivery, but the percentage of transfected cells has been low (Magovern et al., 1996; Mühlausser et al., 1996; Logeart et al., 2001). Hajjar and colleagues (1998) found that briefly clamping both the aorta and the pulmonary artery in rat subjects during adenovirus infusion into the left ventricular (LV) cavity resulted in increased efficiency of adenoviral gene transfer. However, this technique is not clinically applicable because of the risk of myocardial injury. Maurice and colleagues (1999) used a similar technique in the leporine model, whereby a catheter was placed into the LV chamber through the apex of the heart by way of a right thoracotomy (Fig. 4B). The adenovirus solution was rapidly injected while the aorta was cross-clamped for 40 sec. This maneuver enabled all coronary beds to be perfused under pressure. After 6 days, the authors found global myocardial β-galactosidase expression that was evident by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining of both ventricles. Three weeks after gene delivery, however, β2-AR overexpression was minimal, likely attributable to immunological responses to the virus. Better results, according to the authors, were associated with cross-clamping of the aorta and virus administration to both coronary arteries. Kaspar and colleagues (2005), using the same technique with AAV delivery, found that 20–32% of the myocardium expressed enhanced green fluorescent protein (EGFP), which was relatively stable at 1 and 12 months. All the techniques that involve clamping the aorta of a beating heart, however, would be contraindicated in a dilated, failing human heart because this maneuver could theoretically result in irreversible myocardial damage and, in rare cases, myocardial rupture. Furthermore, none of these approaches results in cardiac-selective transgene delivery.
FIG. 4.
(A) Catheter-mediated percutaneous anterograde intracoronary gene delivery. (B) Anterograde intracoronary gene delivery via the left ventricle with short-term aortic cross-clamping in the beating heart.
To optimize a percutaneous intracoronary catheter-based approach in vivo, Logeart and colleagues (2001) tested various procedures, including transient interruption of coronary flow, high-pressure delivery, coronary venous sinus occlusion, and pharmacological agents to increase vessel permeability. They concluded that adenoviral gene transfer to cardiac myocytes was more efficient when the vector residence time and perfusion pressure in the coronary vessels are increased. Later, Emani and colleagues (2003) confirmed these results and showed that efficient and reproducible cardiac transgene expression by intracoronary delivery using adeno-associated viral vectors depends on the infusion flow rate and high perfusion pressure. An intralumenal seal with a catheter balloon inflated to maintain adequate infusion pressure at the time of gene delivery was used. The anterograde infusion of vector at higher flow rates, however, resulted in higher myocardial injury scores.
Hayase and colleagues (2005) combined anterograde coronary gene delivery with coronary artery or coronary vein occlusion to create a brief interruption of coronary blood flow and to prolong exposure to the vector. Quantitative β-galactosidase analysis showed that percutaneous, catheter-based, anterograde, intracoronary gene transfer with coronary occlusion was superior to that without coronary blockade. The authors considered the brief interruption of coronary flow and a high-pressure condition during viral delivery as important to promoting diffuse and homogeneous gene distribution. Collectively, these data indicate that gene transfer during simple, anterograde, direct intracoronary injection is inefficient, due to single-pass kinetics, and the vast majority (> 99%) of vector is delivered into the systemic circulation rather than the myocardium, resulting in collateral organ gene expression (Kaspar et al., 2005; Byrne et al., 2008). Technique optimization mandates additional technical maneuvers such as temporary clamping of the aorta and pulmonary artery, brief interruption of coronary flow, intralumenal sealing of the coronary artery with an inflated balloon, and a high-pressure delivery system. True isolation of the heart by a percutaneous approach, such as by the VFocus (VKardia Inc., Minneapolis, MN) technique, has not been technically feasible (Byrne et al., 2008; Bridges et al., 2009), and the existing methods of intracoronary delivery technique optimization, such as clamping of the aorta or coronary balloon occlusion, have significant associated potential morbidity likely to be prohibitive for clinical application.
Ex Vivo Perfusion
Svensson and colleagues (1999) assessed the efficiency and stability of rAAV-mediated gene transfer in the heart after both intramyocardial injection and intracoronary infusion. Murine hearts were perfused via the left carotid artery with cardioplegic solution at 4°C until they stopped beating. They were then perfused ex vivo for 15 min with AAV-CMV-LacZ. After perfusion, the hearts were transplanted into the neck, and the arterial circulation was reestablished. By 4 weeks after perfusion, ∼40% of the cardiac myocytes were β-galactosidase positive. This level of transduction was stable 8 weeks after perfusion. Shah and colleagues (2000a,b) found that ex vivo intraaortic delivery of adenoviral constructs to donor arrested heart resulted in biventricular myocyte expression of β-galactosidase throughout the myocardium, and β2-adrenergic receptor density was elevated 10-fold in grafts that received adeno-β2-adrenergic receptor. Again, this technique is novel in its application but not likely translatable to clinical application.
Conclusion
On the basis of the reviewed methods, it may be concluded that an optimal, clinically translatable technique for global cardiac myocyte delivery must ideally incorporate the following:
Retrograde transvenous delivery through the coronary sinus or coronary veins (Boekstegers et al., 2000; Boekstegers and Kupatt, 2004; Raake et al., 2004; Bridges et al., 2005; Su et al., 2005) or/and anterograde (Hajjar et al., 1998; Shah et al., 2000a,b; Logeart et al., 2001; Emani et al., 2003)
Extended vector residence time in the coronary circulation (Logeart et al., 2001; Bridges et al., 2002, 2005; Emani et al., 2003; Su et al., 2005)
Increased myocardial transcapillary gradient using physical methods such as increasing perfusion pressure and the flow rate and decreasing the resistance to filtration by increasing endothelial permeability within the coronary circulation with pharmacological agents such as vascular endothelial growth factor (VEGF) or histamine that can enhance transendothelial transport of viral particles from the vasculature into the interstitium (Donahue et al., 1997, 1998; Logeart et al., 2001; Nagata et al., 2001; Wright et al., 2001; Bridges et al., 2005)
Isolation of the cardiac circulation from the systemic circulation to allow for maximization of coronary vector concentration and washout of vector after gene delivery to minimize collateral gene expression with interruption of coronary flow during vector transfer (Bridges et al., 2002, 2005; Byrne et al., 2008)
Removal of blood components (Donahue et al., 1997, 1998; Bridges et al., 2002, 2005)
Minimization of technique-associated morbidity with intracoronary injection (Hou et al., 2003; Raake et al., 2004; Hayase et al., 2005)
Although there does not yet exist one practical, translatable, efficient, and clinically relevant model that has adequately fulfilled all these requirements, the outlook in this exciting era of cardiotherapeutic gene therapy remains promising. With continued focus on optimizing current techniques, the development of vector serotypes with improved cardiac muscle tropism, and the emergence of minimally invasive surgical delivery techniques, cardiac gene therapy is likely to become part of the therapeutic armamentarium for managing an array of cardiovascular diseases including heart failure.
Acknowledgment
This work was sponsored in part by the National Heart Lung and Blood Institute (1-R01-HL083078-01A2), C.R. Bridges, principal investigator.
Author Disclosure Statement
The authors have no proprietary disclosures to report.
References
- Bish L.T. Sleeper M.M. Braibard B. Cole S. Russell N. Withnall E. Arndt J. Reynolds C. Davison E. Sanmiguel J. Wu D. Gao G. Wilson J.M. Sweeney H.L. Percutaneous transendocardial delivery of self-complemen-tary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol. Ther. 2008;16:1953–1959. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- Boekstegers P. von Degenfeld G. Giehrl W. Heinrich D. Hullin R. Kupatt C. Steinbeck G. Baretton G. Middeler G. Katus H. Franz W.M. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7:232–240. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- Boekstegers P. Kupatt C. Current concepts and applications of coronary venous retroinfusion. Basic Res. Cardiol. 2004;99:373–381. doi: 10.1007/s00395-004-0486-3. [DOI] [PubMed] [Google Scholar]
- Bridges C.R. Burkman J.M. Malekan R. Konig S.M. Chen H. Yarnall C.B. Gardner T.J. Stewart A.S. Stecker M.M. Patterson T. Stedman H.H. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann. Thorac. Surg. 2002;73:1939–1946. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- Bridges C.R. Gopal K. Holt D.E. Yarnall C. Cole S. Anderson R.B. Yin X. Nelson A. Kozyak B.W. Wang Z. Lesniewski J. Su L.T. Thesier D.M. Sundar H. Stedman H.H. Efficient myocyte gene delivery with complete cardiac surgical isolation in situ. J. Thorac. Cardiovasc. Surg. 2005;130:1364–1370. doi: 10.1016/j.jtcvs.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Bridges C.R. Recirculating method of cardiac gene delivery should be called “non-recirculating” method. Gene Ther. 2009;16:939–940. doi: 10.1038/gt.2009.35. [DOI] [PubMed] [Google Scholar]
- Buttrick P.M. Kass A. Kitsis R.N. Kaplan M.L. Leinwand L.A. Behavior of genes directly injected into the rat heart in vivo. Circ. Res. 1992;70:193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- Byrne M.J. Power J.M. Preovolos A. Mariani J.A. Hajjar R.J. Kaye D.M. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- Davidson M.J. Jones J.M. Emani S.M. Wilson K.H. Jaggers J. Koch W.J. Milano C.A. Cardiac gene delivery with cardiopulmonary bypass. Circulation. 2001;104:131–133. doi: 10.1161/01.cir.104.2.131. [DOI] [PubMed] [Google Scholar]
- Donahue J.K. Kikkawa K. Johns D.C. Marban E. Lawrence J.H. Ultrarapid, highly efficient viral gene transfer to the heart. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4664–4668. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue J.K. Kikkawa K. Thomas A.D. Marban E. Lawrence J.H. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Ther. 1998;5:630–634. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]
- Emani S.M. Shah A.S. Bowman M.K. Emani S. Wilson K. Glower D.D. Koch W.J. Catheter-based intracoronary myocardial adenoviral gene delivery: Importance of intraluminal seal and infusion flow rate. Mol. Ther. 2003;8:306–313. doi: 10.1016/s1525-0016(03)00149-7. [DOI] [PubMed] [Google Scholar]
- French B.A. Mazur W. Geske R.S. Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- Fromes Y. Salmon A. Wang X. Collin H. Rouche A. Hagège A. Schwartz K. Fiszman M.Y. Gene delivery to the myocardium by intrapericardial injection. Gene Ther. 1999;6:683–688. doi: 10.1038/sj.gt.3300853. [DOI] [PubMed] [Google Scholar]
- Gregorevic P. Blankinship M.J. Allen J.M. Crawford R.W. Meuse L. Miller D.G. Russell D.W. Chamberlain J.S. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P.M. Han Z. Palasis M. Barry J.J. Lederman R.J. Incomplete retention after direct myocardial injection. Catheter Cardiovasc. Interv. 2002;55:392–397. doi: 10.1002/ccd.10136. [DOI] [PubMed] [Google Scholar]
- Guzman R.J. Lemarchand P. Crystal R.G. Epstein S.E. Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ. Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- Gwon H.C. Jeong J.O. Kim H.J. Park S.W. Lee S.H. Park S.J. Huh J.E. Lee Y. Kim S. Kim D.K. The feasibility and safety of fluoroscopy-guided percutaneous intramyocardial gene injection in porcine heart. Int. J. Cardiol. 2001;79:77–88. doi: 10.1016/s0167-5273(01)00410-7. [DOI] [PubMed] [Google Scholar]
- Hajjar R.J. Schmidt U. Matsui T. Guerrero J.L. Lee K.H. Gwathmey J.K. Dec G.W. Semigran M.J. Rosenzweig A. Modulation of ventricular function through gene transfer in vivo. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase M. del Monte F. Kawase Y. MacNeill B.D. McGregor J. Yoneyama R. Hoshino K. Tsuji T. De Grand A.M. Gwathmey J.K. Frangioni J.V. Hajjar R.J. Catheter-based antegrade intracoronary viral gene delivery with coronary venous blockade. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2995–H3000. doi: 10.1152/ajpheart.00703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D. Maclaughlin F. Thiesse M. Panchal V. Bekkers B.C. Wilson E.A. Rogers P.I. Coleman M.C. March K.L. Widespread regional myocardial transfection by plasmid encoding Del-1 following retrograde coronary venous delivery. Catheter Cardiovasc. Interv. 2003;58:207–211. doi: 10.1002/ccd.10417. [DOI] [PubMed] [Google Scholar]
- Ikeda Y. Gu Y. Iwanada Y. Hoshijima M. Oh S.S. Giordano F.J. Chen J. Nigro V. Peterson K.L. Chien K.R. Ross J., Jr. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation. 2002;105:502–508. doi: 10.1161/hc0402.102953. [DOI] [PubMed] [Google Scholar]
- Inagaki K. Fuess S. Storm T.A. Gibson G.A. McTiernan C.F. Kay M.A. Nakai H. Robust systemic transduction with AAV9 in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.M. Koch W.J. Gene therapy approaches to cardiovascular disease. Methods Mol. Med. 2005;112:15–35. doi: 10.1385/1-59259-879-x:015. [DOI] [PubMed] [Google Scholar]
- Jones J.M. Wilson K.H. Koch W.J. Milano C.A. Adenoviral gene transfer to the heart during cardiopulmonary bypass: Effect of myocardial protection technique on transgene expression. Eur. J. Cardiothorac. Surg. 2002;21:847–852. doi: 10.1016/s1010-7940(02)00078-7. [DOI] [PubMed] [Google Scholar]
- Kaspar B.K. Roth D.M. Lai N.C. Drumm J.D. Erickson D.A. McKirnan M.D. Hammond H.K. Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J. Gene Med. 2005;7:316–324. doi: 10.1002/jgm.665. [DOI] [PubMed] [Google Scholar]
- Kizana E. Alexander I.E. Current gene therapeutic potential and current progress. Curr. Gene Ther. 2003;3:418–451. doi: 10.2174/1566523034578249. [DOI] [PubMed] [Google Scholar]
- Kornowski R. Leon M.B. Fuchs S. Vodovotz Y. Flynn M.A. Gordon D.A. Pierre A. Kovesdi I. Keiser J.A. Epstein S.E. Electromagnetic guidance for catheter-based transendocardial injection: A platform for intramyocardial angiogenesis therapy. Results in normal and ischemic porcine models. J. Am. Coll. Cardiol. 2000;35:1031–1039. doi: 10.1016/s0735-1097(99)00642-7. [DOI] [PubMed] [Google Scholar]
- Lamping K.G. Rios C.D. Chun J.A. Ooboshi H. Davidson B.L. Heistad D.D. Intrapericardial administration of adenovirus for gene transfer. Am. J. Physiol. 1997;272:H310–H317. doi: 10.1152/ajpheart.1997.272.1.H310. [DOI] [PubMed] [Google Scholar]
- Lin H. Parmacek M.S. Morle G. Bolling S. Leiden J.M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Logeart D. Hatem S.N. Heimburger M. Roux A.L. Michel J.B. Mercadier J.J. How to optimize in vivo gene transfer to cardiac myocytes: Mechanical or pharmacological procedures? Hum. Gene. Ther. 2001;12:1601–1610. doi: 10.1089/10430340152528101. [DOI] [PubMed] [Google Scholar]
- Losordo D.W. Vale P.R. Symes J. Dunnington C. Esakof D. Maysky M. Ashare A.B. Lathi K. Isner J.M. Gene therapy for myocardial angiogenesis: Initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- Ly H. Kawase Y. Yoneyamam Hajjar R.J. Gene therapy in the treatment of heart failure. Physiology. 2007;22:81–96. doi: 10.1152/physiol.00037.2006. [DOI] [PubMed] [Google Scholar]
- Lyon A.R. Sato M. Hajjar R.J. Samulski R.J. Harding S.E. Gene therapy: Targeting the myocardium. Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- Magovern C.J. Mack C.A. Zhang J. Hahn R.T. Ko W. Isom O.W. Crystal R.G. Rosengart T.K. Direct in vivo gene transfer to canine myocardium using a replication-deficient adenovirus vector. Ann. Thorac. Surg. 1996;62:425–433. [PubMed] [Google Scholar]
- March K.L. Woody M. Mehdi K. Zipes D.P. Brantly M. Trapnell B.C. Efficient in vivo catheter-based pericardial gene transfer mediated by adenoviral vectors. Clin. Cardiol. 1999;22:123–129. doi: 10.1002/clc.4960221308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice J.P. Hata J.A. Shah A.S. White D.C. McDonald P.H. Dolber P.C. Wilson K.H. Lefkowitz R.J. Glower D.D. Koch W.J. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary β2-adrenergic receptor gene delivery. J. Clin. Invest. 1999;104:21–29. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlausser J. Jones M. Yamada I. Cirielli C. Lemarchand P. Gloe T.R. Bewig B. Signoretti S. Crystal R.G. Capogrossi M.C. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther. 1996;3:145–153. [PubMed] [Google Scholar]
- Nagata K. Marban E. Lawrence J.H. Donahue J.K. Phosphodiesterase inhibitor-mediated potentiation of adenovirus delivery to myocardium. J. Mol. Cell Cardiol. 2001;33:575–580. doi: 10.1006/jmcc.2000.1322. [DOI] [PubMed] [Google Scholar]
- Ohshima S. Shin J.H. Yuasa K. Nishiyama A. Kira J. Okada T. Takeda S. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscule. Mol. Ther. 2009;17:73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raake P. von Degenfeld G. Hinkel R. Vachenauer R. Sandner T. Beller S. Andrees M. Kupatt C. Schuler G. Boekstegers P. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. J. Am. Coll. Cardiol. 2004;44:1124–1129. doi: 10.1016/j.jacc.2004.05.074. [DOI] [PubMed] [Google Scholar]
- Rosengart T.K. Lee L.Y. Patel S.R. Kligfield P.D. Okin P.M. Hackett N.R. Isom O.W. Crystal R.G. Six-month assessment of a phase І trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration an adenovirus vector expressing the VEGF121 cDNA. Ann. Surg. 1999;230:466–472. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn T.A. Hackett N.R. Lee L.Y. El-Sawy T. Blanco I. Tarazona N. Deutsch E. Crystal R. Rosengart T.K. Percutaneous endocardial transfer and expression of genes to the myocardium utilizing fluoroscopic guidance. Catheter Cardiovasc. Interv. 2001;52:260–266. doi: 10.1002/1522-726x(200102)52:2<260::aid-ccd1061>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Shah A.S. Lilly E. Kypson A.P. Tai O. Hata J.A. Pippen A. Silvestry S.C. Lefkowitz R.J. Glower D.D. Koch W.J. Intracoronary adenovirus-mediated delivery and overexpression of the β2-adrenergic receptor in the heart: Prospects for molecular ventricular assistance. Circulation. 2000a;101:408–414. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- Shah A.S. White D.C. Tai O. Hata J.A. Wilson K.H. Pippen A. Kypson A.P. Glower D.D. Lefkowitz R.J. Koch W.J. Adenovirus-mediated genetic manipulation of the myocardial β-adrenergic signaling system in transplanted hearts. J. Thorac. Cardiovasc. Surg. 2000b;120:581–588. doi: 10.1067/mtc.2000.107519. [DOI] [PubMed] [Google Scholar]
- Svensson E.C. Marshall D.J. Woodard K. Lin H. Jiang F. Chu L. Leiden J.M. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation. 1999;99:201–205. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- Sylvén C. Sarkar N. Insulander P. Kennebäck G. Blomberg P. Islam K. Drvota V. Catheter-based transendocardial myocardial gene transfer. J. Interv. Cardiol. 2002;15:7–13. doi: 10.1111/j.1540-8183.2002.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Su L.T. Gopal K. Wang Z. Yin X. Nelson A. Kozyak B.W. Burkman J.M. Mitchell M.A. Low D.W. Bridges C.R. Stedman H.H. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112:1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- Swain J.D. Katz M.G. Fargnoli A. White J. Thesier D.M. Yarnall C. Isidro A. Petrov M. Holt D. Nolen-Walston R. Stedman H. Koch W.J. Pilla J.J. Rabinowitz J. Bridges C.R. Improved myocardial mechanics after GRK2 inhibition using molecular cardiac surgery with recirculating delivery (MCARD™) to deliver AAV6-βARKct in sheep. Circ. Res. 2009;105:e58. [Google Scholar]
- Tomiyasu K. Oda Y. Nomura M. Satoh E. Fushiki S. Imanishi J. Kondo M. Mazda O. Direct intra-cardiomuscular transfer of β2-adrenergic receptor gene augments cardiac output in cardiomyopathic hamsters. Gene Ther. 2000;7:2087–2093. doi: 10.1038/sj.gt.3301329. [DOI] [PubMed] [Google Scholar]
- Vale P.R. Losordo D.W. Tkebuchava T. Chen D. Milliken C.E. Isner J.M. Catheter-based myocardial gene transfer utilizing nonfluoroscopic electromechanical left ventricular mapping. J. Am. Coll. Cardiol. 1999;34:246–254. doi: 10.1016/s0735-1097(99)00143-6. [DOI] [PubMed] [Google Scholar]
- Vinge L.E. Raake P.W. Koch W.J. Gene therapy in heart failure. Circ. Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Harsdorf R. Schott R.J. Shen Y.T. Vatner S.F. Mahdavi V. Nadal-Ginard B. Gene injection into canine myocardium as a useful model for studying gene expression in the heart of large mammals. Circ. Res. 1993;72:688–695. doi: 10.1161/01.res.72.3.688. [DOI] [PubMed] [Google Scholar]
- Wang Z. Zhu T. Qiao C. Zhou L. Wang B. Zhang J. Chen C. Li J. Xiao X. Adeno-associated virus, serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- White J. Thesier D. Henderson A. Swain J. Katz M. Yarnall C. Isidro A. Chen H. Holt D. Farag J. Petrov M. Soltys S. Stedman H. Rabinowitz J. Bridges C.R. Molecular cardiac surgery: A translatable, highly efficient, global scAAV-6-mediated gene delivery technique to the ovine myocardium. Mol. Ther. 2009a;17(Suppl. 1):S27–S28. [Google Scholar]
- White J. Swain J.D. Katz M. Thesier D. Henderson A. Yarnell C. Isidro A. Holt D. Mead A. Farag J. Soltys S. Culp W. Nolen-Walston R. Norton J. Tomasulo K. Petrov M. Stedman H. Rabinowitz J. Bridges C.R. Adeno-associated viral vector-mediated gene transfer to the heart using molecular cardiac surgery: A novel translatable closed recirculation system for myocardial gene delivery. (Or 12 abstract) Hum. Gene Ther. 2009b;20:393–394. [Google Scholar]
- Wright M.J. Wightman L.M.L. Latchman D.S. Marber M.S. In vivo myocardial gene transfer: Optimization and evaluation of intracoronary gene delivery in vivo. Gene Ther. 2001;8:1833–1839. doi: 10.1038/sj.gt.3301614. [DOI] [PubMed] [Google Scholar]
- Zhang J.C.L. Woo Y.J. Chen J.A. Swain J.L. Sweeney H.L. Efficient transmural cardiac gene transfer by intrapericardial injection in neonatal mice. J. Mol. Cell Cardiol. 1999;31:721–732. doi: 10.1006/jmcc.1998.0905. [DOI] [PubMed] [Google Scholar]