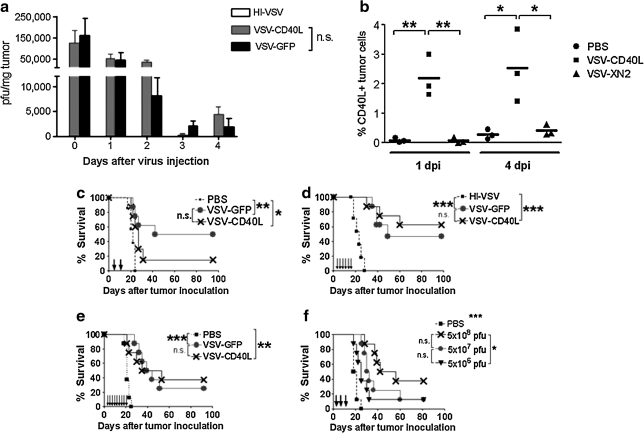
FIG. 2.
Intratumoral administration of VSV-CD40L into established subcutaneous B16ova tumors in C57BL/6 mice. (a) C57BL/6 mice (n = 3 mice per group per time point) bearing 7-day-old B16ova tumors were given one intratumoral injection of VSV at a dose of 5 × 108 PFU. Tumors were collected and snap-frozen in liquid nitrogen at various times postinfection, and titrated for VSV by virus plaque assay. (b) Similar to (a), VSV-injected B16ova tumors (three mice per group) were harvested 1 and 4 days after injection, disrupted to obtain single-cell suspensions, stained with PE-conjugated anti-CD40L, and subjected to flow cytometric analysis. dpi, days postinjection. (c–e) B16ova melanoma tumors were established subcutaneously in C57BL/6 mice 7 days before intratumoral viral administration. Either VSV-GFP or VSV-CD40L (5 × 108 PFU) was injected two times (c), six times (d), and nine times (e). Tumor growth and overall survival were monitored (n = 8 mice per group). (f) Dose–response survival curve using VSV-CD40L. Three intratumoral injections of recombinant VSVs were administered into subcutaneous B16ova tumors at various doses: 5 × 108, 5 × 107, and 5 × 106 PFU given every other day. n.s., not significant. *p < 0.05, **p < 0.01, ***p < 0.001.