Abstract
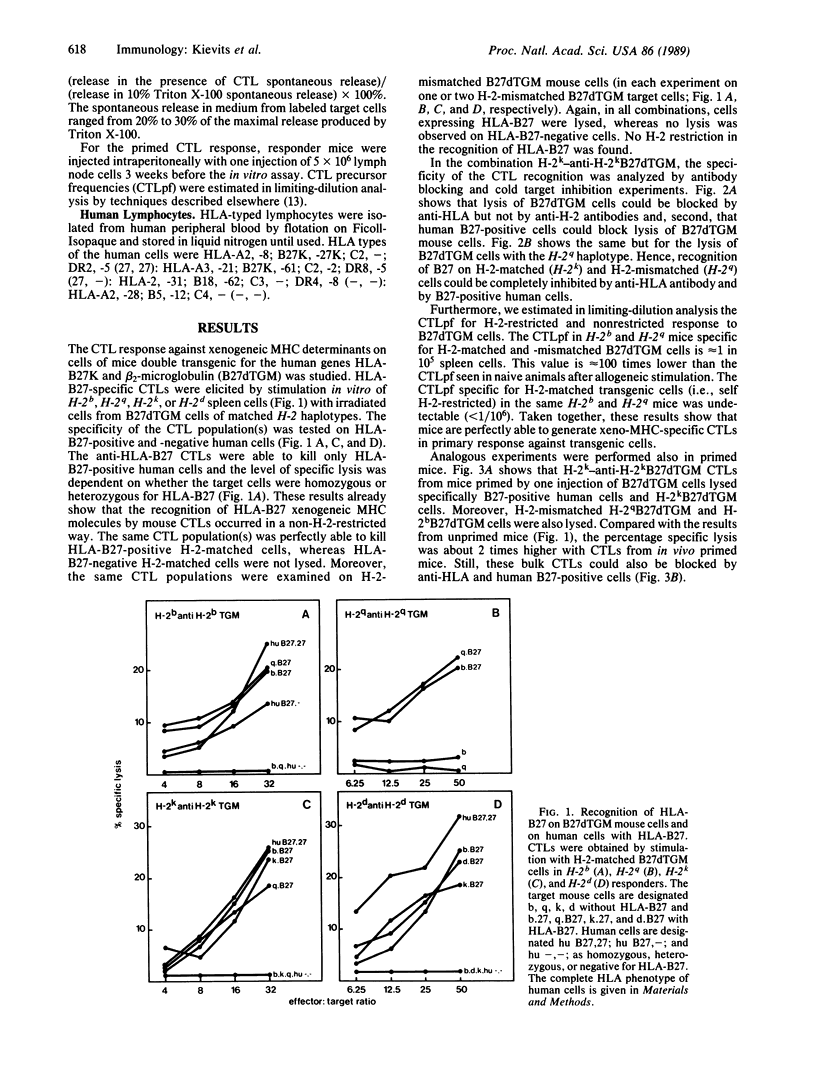
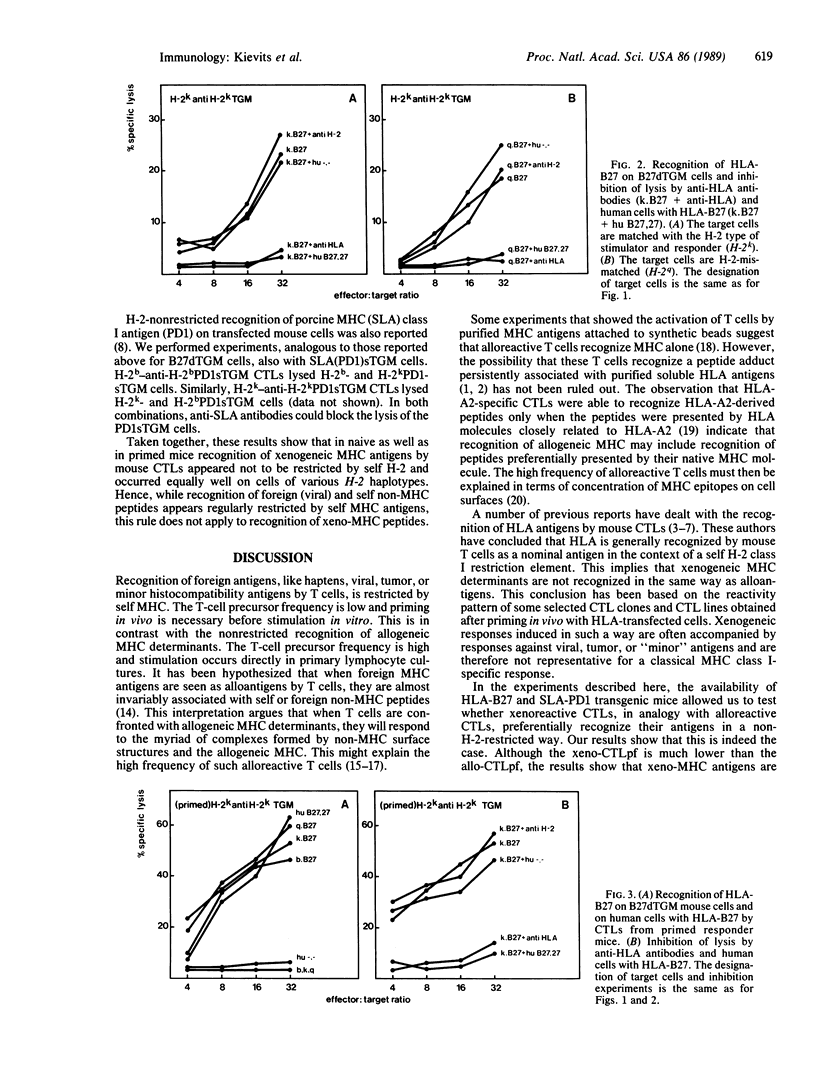
Cytotoxic T lymphocytes (CTLs) recognize antigens in the context of major histocompatibility complex (MHC) class I gene products. The T-cell receptor (TCR) that mediates this MHC-restricted antigen recognition recognizes short peptide fragments rather than the intact antigen. Presentation of peptides to the TCR may thus be a major function of the MHC. An intriguing question emerging from this model is whether peptide presentation also applies to foreign MHC antigens and which of the available MHC molecules can present preferentially the peptides of the foreign MHC molecule. Allo- and xenoreactive CTLs might either recognize native MHC class I molecules or peptides presented by self MHC or by the foreign class I MHC itself. The finding that synthetic peptides corresponding to MHC class I regions are recognized by allo- and xenoreactive CTLs suggests that recognition of foreign MHC by CTLs might involve degraded fragments presented by syngeneic class I molecules. We used MHC transgenic mice as a tool to study these questions. The CTL responses against human (HLA) antigen B27 were analyzed by using HLA-B27 transgenic mice with various H-2 haplotypes. We report here that mouse xeno-MHC-specific (anti-B27) CTLs are perfectly able to kill human and mouse cells expressing the appropriate xenoantigen and that in primary and secondary responses to xeno-MHC, the mouse T-cell repertoire does not use self-H-2 as a restriction element. Absence of H-2 restriction was confirmed by the lack (less than 1/10(6] of H-2-restricted HLA-specific CTL precursors. Therefore, H-2-restricted recognition of xeno-MHC antigens cannot be generalized as part of a classical MHC class I-specific response. These results indicate that xenoreactive CTLs usually recognize intact MHC molecules or MHC peptides preferentially presented by their native MHC molecule. We suggest the latter possibility.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achour A., Begue B., Gomard E., Paul P., Sayagh B., Van Pel A., Levy J. P. Specific lysis of murine cells expressing HLA molecules by allospecific human and murine H-2-restricted anti-HLA T killer lymphocytes. Eur J Immunol. 1986 Jun;16(6):597–604. doi: 10.1002/eji.1830160603. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. Killer cells reactive to altered-self antigens can also be alloreactive. Proc Natl Acad Sci U S A. 1977 May;74(5):2094–2098. doi: 10.1073/pnas.74.5.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Pescovitz M. D., Frels W. I., Singer D. S., Hodes R. J. Cytotoxic T lymphocyte recognition of a xenogeneic major histocompatibility complex antigen expressed in transgenic mice. Eur J Immunol. 1987 Jul;17(7):1035–1041. doi: 10.1002/eji.1830170721. [DOI] [PubMed] [Google Scholar]
- Clayberger C., Parham P., Rothbard J., Ludwig D. S., Schoolnik G. K., Krensky A. M. HLA-A2 peptides can regulate cytolysis by human allogeneic T lymphocytes. Nature. 1987 Dec 24;330(6150):763–765. doi: 10.1038/330763a0. [DOI] [PubMed] [Google Scholar]
- Cytolytic thymus-derived lymphocytes specific for allogeneic stimulator cells crossreact with chemically modified syngeneic cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1229–1233. doi: 10.1073/pnas.74.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. The requirements for antigen multivalency in class I antigen recognition and triggering of primed precursor cytolytic T lymphocytes. J Immunol. 1986 Apr 15;136(8):2816–2825. [PubMed] [Google Scholar]
- Holterman M. J., Engelhard V. H. HLA antigens expressed on murine cells are preferentially recognized by murine cytotoxic T cells in the context of the H-2 major histocompatibility complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9699–9703. doi: 10.1073/pnas.83.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W. M., Boog C. J., Roep B. O., Voordouw A. C., Melief C. J. Failure or success in the restoration of virus-specific cytotoxic T lymphocyte response defects by dendritic cells. J Immunol. 1988 May 1;140(9):3186–3193. [PubMed] [Google Scholar]
- Kievits F., Ivanyi P., Krimpenfort P., Berns A., Ploegh H. L. HLA-restricted recognition of viral antigens in HLA transgenic mice. Nature. 1987 Oct 1;329(6138):447–449. doi: 10.1038/329447a0. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Chaouat G., Rabourdin-Combe C., Claverie J. M. Working principles in the immune system implied by the "peptidic self" model. Proc Natl Acad Sci U S A. 1987 May;84(10):3400–3404. doi: 10.1073/pnas.84.10.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimpenfort P., Rudenko G., Hochstenbach F., Guessow D., Berns A., Ploegh H. Crosses of two independently derived transgenic mice demonstrate functional complementation of the genes encoding heavy (HLA-B27) and light (beta 2-microglobulin) chains of HLA class I antigens. EMBO J. 1987 Jun;6(6):1673–1676. doi: 10.1002/j.1460-2075.1987.tb02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. T cells can distinguish between allogeneic major histocompatibility complex products on different cell types. Nature. 1988 Apr 28;332(6167):840–843. doi: 10.1038/332840a0. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Accolla R. S., Jordan B. H2-restricted recognition of cloned HLA class I gene products expressed in mouse cells. J Immunol. 1986 Jun 15;136(12):4340–4347. [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Corradin G., Jordan B. R., Cerottini J. C. H-2-restricted cytolytic T cells specific for HLA can recognize a synthetic HLA peptide. Nature. 1986 Dec 11;324(6097):578–579. doi: 10.1038/324578a0. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Bevan M. J. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977 Mar 1;29(1):1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- Opolski A., Kievits F., Iványi P. Polymorphic and autoreactive H-2-specific monoclonal antibody isolated after injections of syngeneic Sendai virus-coated lymphocytes. Immunogenetics. 1986;24(6):402–408. doi: 10.1007/BF00377959. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984 Jul;133(1):368–375. [PubMed] [Google Scholar]


