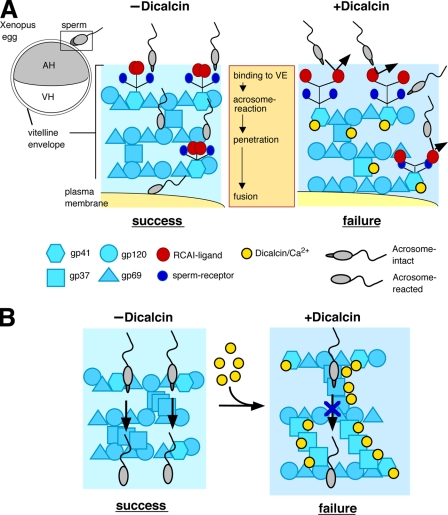
FIGURE 9.
Schematic models of the inhibitory action of dicalcin on fertilization in X. laevis. A, glycoprotein model that involves allosteric conformational change of gp41 caused by dicalcin. In a currently favored model in Xenopus egg, acrosome-intact sperm bind to gp41 (a frog orthologue of mouse ZP3; a major binding partner of sperm) and gp69/64 (an orthologue of mouse ZP2) via carbohydrate moieties (sperm receptors). After acrosome reaction, sperm penetrate through the VE and fuse with an egg (−Dicalcin). The Ca2+-bound form of dicalcin binds to gp41 (and gp37 additionally), leading to a conformational change that could cause an exposure of RCAI ligands. This allosteric change in the configuration of oligosaccharides may mask sperm receptors, forming a functional barrier to prevent sperm binding, penetration, and fusion (+Dicalcin). AH, animal hemisphere; VH, vegetal hemisphere. B, VE structure model that involves structural change in the VE framework caused by dicalcin. VE proteins (gp37, gp41, gp69/64, and gp120) associate with each other and constitute the filament of VE (depicted in −Dicalcin; modified from the schematic model in mammalian ZP). Dicalcin binding to gp37 and gp41 caused a structural change in the three-dimensional structure of the filamentous VE network. This change may involve disorganization of filaments, including fasciculation of filaments, and cause enlargement of the pore size among VE filaments as depicted in +Dicalcin. Symbols are the same in A and B.