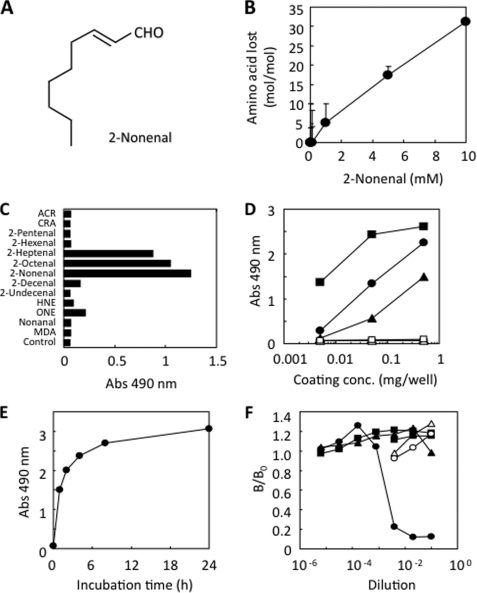
FIGURE 1.
Monoclonal antibody against protein-bound 2-nonenal. A, chemical structure of 2-nonenal. B, loss of lysine residues in protein treated with 2-nonenal. HSA (1 mg/ml) was incubated with 0–10 mm 2-nonenal in 50 mm sodium phosphate buffer, pH 7.2, at 37 °C. C, immunoreactivity of mAb 27Q4 to aldehyde-modified proteins. Affinity of mAb 27Q4 was determined by a direct ELISA using aldehyde-modified BSA as the absorbed antigen. A coating antigen (0.5 μg/well) was prepared by incubating 1 mg of BSA with 1 mm aldehydes in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C. ACR, acrolein; CRA, crotonaldehyde; MDA, malondialdehyde; HNE, 4-hydroxy-2-nonenal; ONE, 4-oxo-2-nonenal. D, immunoreactivity of mAb 27Q4 to 2-nonenal-modified proteins. A coating antigen (0.005–0.5 μg/well) was prepared by incubating 1 mg of protein with 1 mm 2-nonenal in 1 ml of 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C. Proteins are as follows: open square, native lactoglobulin; closed triangle, 2-nonenal-modified HSA; closed circle, 2-nonenal-modified BSA; closed square, 2-nonenal-modified lactoglobulin. E, time-dependent increase in the immunoreactivity of protein treated with 2-nonenal. HSA (1 mg/ml) was incubated with 1 mm 2-nonenal in 50 mm sodium phosphate buffer, pH 7.2, at 37 °C. F, competitive ELISA analysis with the reaction mixtures of amino acid derivatives and 2-nonenal. Competitors were prepared by incubating 50 mm amino acid derivatives, Nα-acetyllysine, Nα-acetylhistidine, or Nα-acetylcysteine, in the presence or absence of 2-nonenal (50 mm), in 50 mm sodium phosphate buffer, pH 7.2, for 24 h at 37 °C. Competitors are as follows: open circle, Nα-acetyllysine; open triangle, Nα-acetylhistidine; open square, Nα-acetylcysteine; closed circle, 2-nonenal/Nα-acetyllysine; closed triangle, 2-nonenal/Nα-acetylhistidine; closed square, 2-nonenal/Nα-acetyllysine.