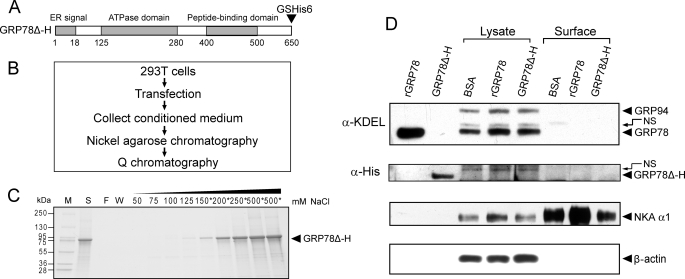
FIGURE 10.
Extracellular GRP78 does not stably bind to cell surface. A, schematic illustration of GRP78Δ-H mutant encoded by expression plasmid, in which KDEL motif was deleted and glycine (G) and serine (S), followed by six tandem histidine (GSHis6), were added at the C terminus of GRP78. B, methodological flow chart of expression and purification of secreted GRP78Δ-H. C, Q chromatography purification for GRP78Δ-H. Protein purified by nickel-agarose affinity chromatography was loaded onto a Q column, and after extensive wash of the column, elution was performed with buffer containing the indicated concentration (50–500 mm) of NaCl. All fractions were run on SDS-PAGE followed by Coomassie Blue staining. The fractions marked with an asterisk were collected and saved. The molecular weight of protein marker (M) is indicated on the left. S, protein initially loaded onto the Q column; F, flow-through fraction; W, wash fraction. D, detection of extracellular GRP78 entering or binding to surface of 293T cells. 10 μg of rGRP78, purified GRP78Δ-H, or bovine serum albumin (BSA) were added in the medium of 293T cells in 6-well dishes, respectively, and incubated for 24 h. The cell surface and 20% lysate input of GRP78 and GRP78Δ-H were detected by Western blot against either anti-KDEL or anti-His antibody. 1 μg of rGRP78 and purified GRP78Δ-H were loaded as positive control. The upper band above the positive control is a nonspecific (NS) band. Sodium-potassium-ATPase (NKA α1) served as cell surface protein loading control. β-Actin was used as lysate input loading control.