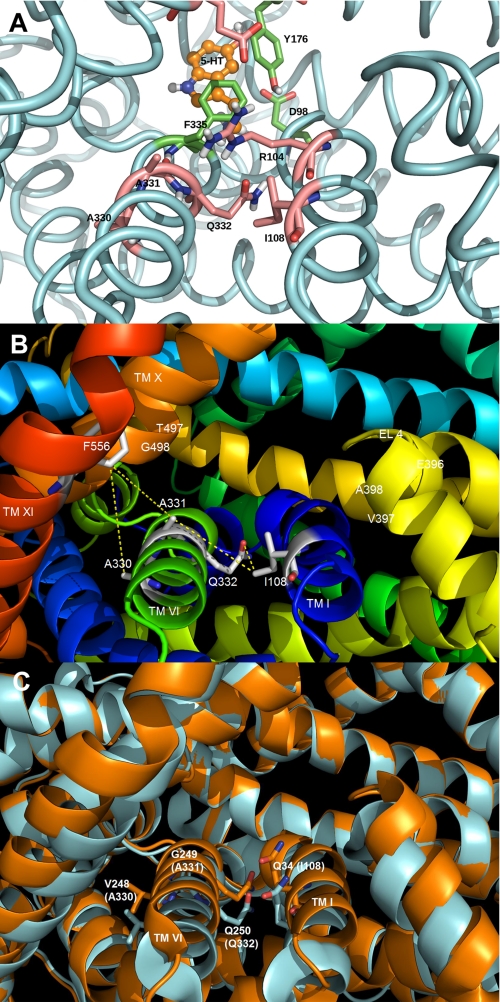
FIGURE 1.
Homology model of the SERT demonstrating the presence of residues predicted to be at the entrance to the permeation pore. The SERT TMHs predicted to form the entrance to the permeation pathway (TMHs I, VI, X, XI, and EL4) are shown. A, homology model of SERT based on the closed-closed conformation of LeuTAa (5) showing the predicted location of the 5-HT substrate binding site. The protein backbone is colored light blue, and three residues in the substrate pocket (Tyr176, Phe335, and Asp98) are colored green. The residues above this pocket, in the extracellular facing vestibule, are colored pink and include Ala330, Ala331, Gln332, Ile108, and the gating residues Arg104 and Glu493. B, SERT homology model with the residues (labeled in white) predicted to be positioned at the extracellular surface of the transporter. Each residue was computationally mutated to cysteine, and the approximate distance (in Å) between the Cys pair (S–S) was determined using PyMOL. PyMOL was allowed to position the -SH side chain in the default orientation with no steric clashes observed. C, overlay of different conformations of LeuTAa. Closed-closed conformation is shown in blue, whereas the tryptophan-bound LeuTAa structure is shown in orange (30). Residues corresponding to hSERT Ile108, Ala330, Ala331, and Gln332 are shown in stick form on TMH I and VI, respectively. The structures reveal significant movement of TMHs I and VI as a result of the ligand (tryptophan) binding.