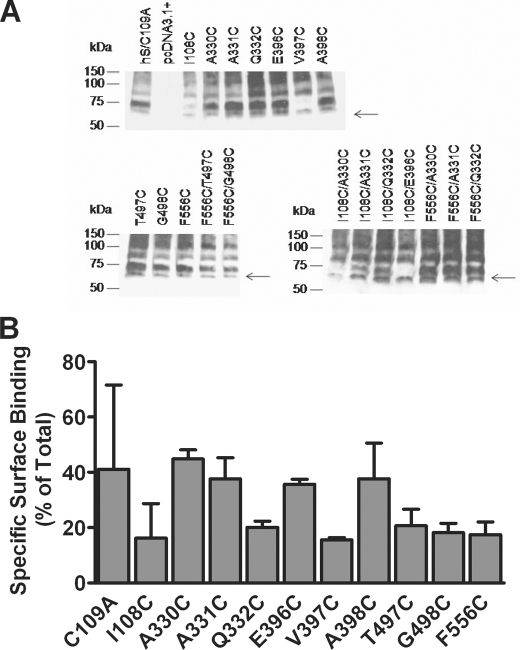
FIGURE 3.
Evaluation of hSERT Cys mutant protein expression. A, whole cell lysates from HEK-293 cells transiently transfected with the hSERT C109A mutant, hSERT Cys single mutants, and hSERT Cys double mutants were prepared as described under “Experimental Procedures.” Proteins were separated by SDS-PAGE, and the transporter was detected by immunoblotting using an hSERT monoclonal antibody. The arrows denote hSERT migration at 65 kDa. A more highly glycosylated form migrates at ∼75–80 kDa. The blots are representative of three independent experiments. B, cell surface radioligand binding for wild-type or hSERT mutants. Assays were performed in 24-well plates coated with poly-d-lysine as described under “Experimental Procedures.” A saturating concentration of [3H]citalopram (20 nm) was used for hSERT and mutants. Nonspecific binding was defined as the binding of radiolabeled ligand in the presence of 10 μm fluoxetine. Internal binding was determined as the binding in the presence of 400 μm MPP+. Specific surface binding was calculated as (total binding − binding in the presence fluoxetine) − (binding in the presence of MPP+ − binding in the presence of fluoxetine). Transporter molecules were calculated assuming one citalopram bound per transporter. Bars, the mean of three independent experiments ± S.E. (error bars).