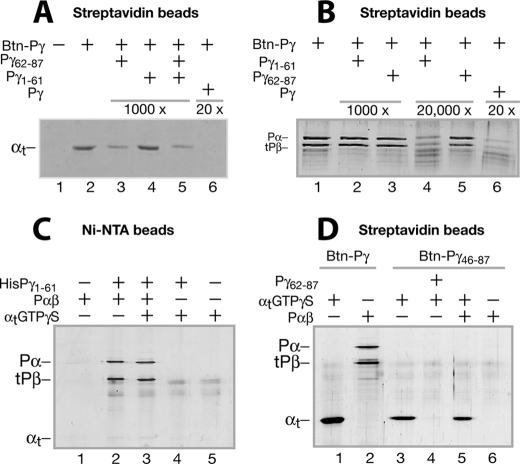
FIGURE 3.
Differential Pγ domain interactions with αtGTPγS and Pαβ evidenced by pull-down experiments. Pull-down experiments were performed with Btn-Pγ or Btn-Pγ(46–87) immobilized on strepavidin beads or HisPγ(1–61) immobilized on Ni2+-nitrilotriacetic acid beads. Conditions are described under “Experimental Procedures” unless otherwise stated. Trypsin inhibitor instead of BSA was included to block nonspecific protein-bead interactions because BSA (66 kDa) migrates too close to tPβ (∼70 kDa) on the gel. The gels in A–D each represent at least three similar experiments. A, pull-down of αtGTPγS by Btn-Pγ. The control without Btn-Pγ is shown in lane 1. The Pγ peptide Pγ(62–87) (lane 3), Pγ(1–61) (lane 4), or both (lane 5) in a 1000-fold molar excess over Btn-Pγ or the full-length Pγ in a 20-fold excess (lane 6) was added to compete with the Btn-Pγ-αtGTPγS interaction. B, pull-down of Pαβ by Btn-Pγ. Pγ(1–61) (lanes 2 and 4) or Pγ(62–87) (lanes 3 and 5), in a 1000- or 20,000-fold molar excess over Btn-Pγ, was present, competing with the Btn-Pγ-Pαβ interaction. C, pull-down of Pαβ or αtGTPγS using the HisPγ(1–61) peptide immobilized to nickel beads. Lanes 1 and 5, controls without HisPγ(1–61) for pulling down Pαβ (lane 2) and αtGTPγS (lane 4), respectively. Both Pαβ and αtGTPγS were added in the reaction of lane 3. D, pull-down of αtGTPγS (lanes 3 and 4) or Pαβ (lane 6) by Btn-Pγ(46–87). Pγ(62–87) in a 200-fold molar excess over Btn-Pγ(46–87) was used to compete with the Btn-Pγ(46–87)-αtGTPγS interaction. Pull-down of αtGTPγS (lane 1) or Pαβ (lane 2) by the full-length Btn-Pγ was also performed to compare with the Btn-Pγ(46–87) pull-down conditions.