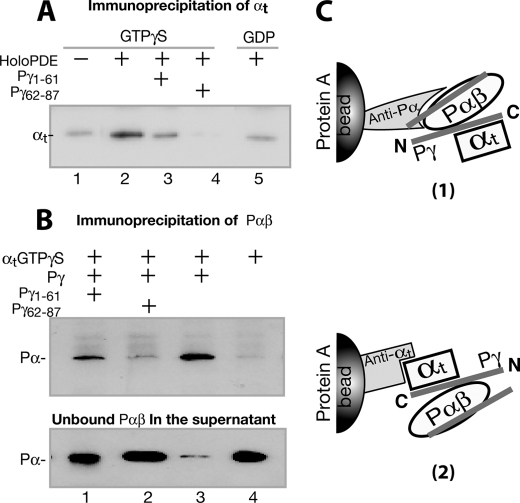
FIGURE 5.
Pγ-dependent αt·PDE6 interaction detected through immunoprecipitation. The immunoprecipitation experiments were conducted using purified proteins, antibodies, and Protein A beads, as described under “Experimental Procedures.” The blots shown in the figure each represent at least three similar experiments. A, αtGTPγS was immunoprecipitated by the holo-PDE6·anti-Pα·Protein A beads (lane 2). Lane 1, the control with no holo-PDE6. Pγ peptide Pγ(1–61) (lane 3) or Pγ(62–87) (lane 4) in a 200-fold molar excess was added to compete with the Pγ interactions. In lane 5, αtGDP instead of αtGTPγS was used. B, Pαβ was immunoprecipitated by the αtGTPγS·anti-αt·Protein A beads (lane 3). Lane 4 is the control in the absence of Pγ. In lanes 1 and 2, Pγ(1–61) and Pγ(62–87) of 200-fold molar excess were added, respectively, to compete with the Pγ interactions. Immunoblotting of supernatants containing unbound Pαβ is shown in the bottom panel. C, diagrams depicting the experimental strategies in A and B. For simplicity, the second αt molecule that could also bind to Pγ·Pαβ (46) is not shown.