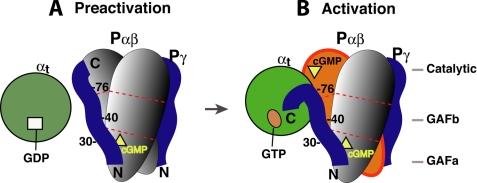
FIGURE 7.
Schematic diagram of the complementary Pγ interactions with its two targets during PDE6 activation. The molecules involved in PDE6 activation are represented by different shapes. Egg, Pα and Pβ; circle, αt; ribbon, Pγ; square, GDP; oval, GTP; triangle, cGMP. For simplicity, the three domains of Pα and Pβ are shown as segments separated by the dotted red lines. Our previous studies revealed that the Pγ Phe30 region preferred binding to Pα, whereas the Ser40 region favored binding to Pβ, suggesting simultaneous Pγ interactions with Pα and Pβ (16, 28). Because PDE6 can only be efficiently activated 50% by transducin (26, 46), it is highly likely that only one Pγ is displaced by αtGTP during PDE6 activation in the mammalian retina, whereas the other Pγ (on the opposite side) stays tightly bound to Pαβ (1). A movement of the Pγ(78–87) segment from Pαβ to αtGTP was suggested by Barren et al. (5), based on the crystal structure of the Pγ(70–87)·PDE5/6 catalytic domain complex.