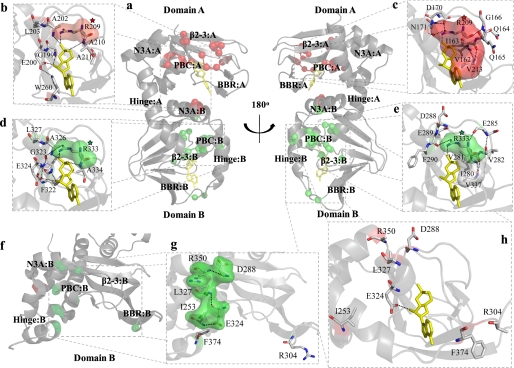
FIGURE 2.
Map of significant mutation-induced chemical shift changes onto the crystal structures of the R-subunit. a, shown are chemical shift changes (red spheres, R209K; green spheres, R333K) mapped onto the cAMP-bound state of the R-subunit. Chemical shift changes observed within the PBC of domains A (b) and B (d) as well as the β2-3 loop of domains A (c) and B (e) can largely be explained by the extensive hydrogen bond networks within the cAMP-bound structure. f, chemical shift changes (red spheres, R209K; green spheres, R333K) mapped onto domain B of the C-subunit-bound state of the R-subunit are shown. Several residues that appear unrelated in the cAMP-bound structure (g) form a series of interactions within the C-subunit-bound state (h). Surfaces are shown for selected residues to indicate hydrophobic contacts (panels b–e) or to show clustering of residues that form a hydrogen bond network in the C-subunit bound state (panel g). Surfaces for residues that show chemical shift changes in response to the R209K or R333K mutations are shown as red and green, respectively, whereas surfaces shown in white belong to residues that do not show a significant chemical shift change in response to either mutation. The red- and green- colored stars are shown to indicate the conserved Arg mutation in CBD-A or CBD-B, respectively. Molecules of cAMP are shown as yellow sticks.