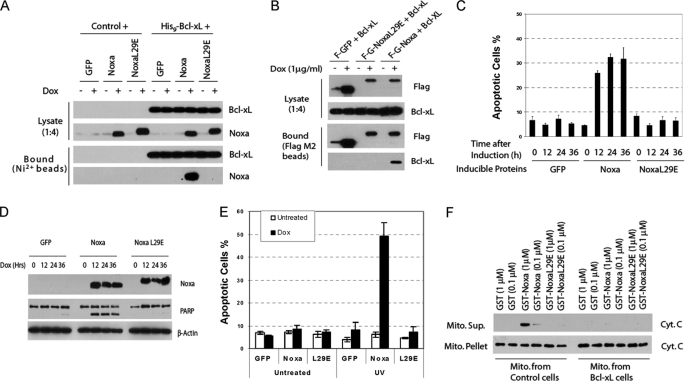
FIGURE 5.
BH3-dependent Bcl-xL binding, cytochrome release, and apoptosis induction by Noxa. A, pulldown of Noxa by Bcl-xL is BH3-dependent. HeLa cells expressing the indicated proteins under the control of tetracycline (Tet-on) were infected with a retrovirus expressing either vector or His9-Bcl-xL. After induction with Dox (1 μg/ml) for 8 h, cell lysates were subjected to nickel bead pulldown as described in Fig. 3E. B, pulldown of Bcl-xL by Noxa is BH3-dependent. HeLa cells expressing FLAG-GFP, FLAG-GFP-Noxa, or FLAG-GFP-NoxaL29E under the control tetracycline were infected with a retrovirus expressing either vector or Bcl-xL. After Dox induction, cell lysates were subjected to nickel bead pulldown as described in Fig. 3E. C, BH3-dependent apoptotic activity of Noxa is shown. The Tet-on HeLa cells expressing GFP, Noxa, or NoxaL29E were induced by Dox for the indicated times. Apoptosis was quantified by Hoechst staining. The results are the mean ± S.D. from at least three independent experiments. D, BH3-dependent PARP cleavage activity of Noxa is shown. Cell lysates from C were analyzed by Western blot. E, sensitization to UV-induced apoptosis by Noxa is shown. The Tet-on HeLa cells expressing GFP, Noxa, or NoxaL29E were induced by Dox for 3 h before being subjected to UV (20 J/m2) treatment. Six hours later, apoptosis was quantified by Hoechst staining. The results are the mean ± S.D. from two independent experiments. F, BH3-dependent in vitro cytochrome c release activity of Noxa is shown. Mitochondria were isolated from regular or Bcl-xL-overexpressing HeLa cells. Recombinant GST or GST-Noxa fusion protein (supplemental Fig. S4) was incubated with mitochondria at 30 °C for 1 h. The supernatant (Sup.) and the mitochondrial (Mito.) pellets were analyzed by Western blot using a cytochrome c antibody.