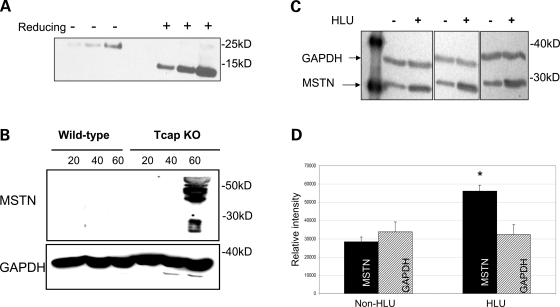
Figure 5.
Myostatin protein expression is increased in Tcap KO mouse muscles. Immunoblots were performed on muscle lysates from WT and Tcap KO gastrocnemius muscles probed with an anti-myostatin antibody. (A) To evaluate the myostatin antibody, recombinant purified human myostatin protein was subjected to both non-reducing (−) and reducing (+) conditions as indicated. One hundred and twenty-five, 250 and 500 ng purified myostatin protein were loaded in each condition. Bands at ∼26 and ∼13 kDa were clearly seen, indicating the sensitivity of the antibody to detect intact and cleaved C-terminal dimer myostatin proteins, respectively. (B) Both the 45-kDa precursor and 26-kDa processed myostatin bands were observed, but only in Tcap KO muscle lysates and not in WT muscles. (C) To assess the sensitivity of myostatin antibody in skeletal muscle tissue, female rats (n = 3) were suspended by their tails (HLU) for four weeks, SOL muscles were subsequently removed and muscle lysates were probed with anti-myostatin antibody. Intensity of myostatin bands (∼26 kDa) increased relative to GAPDH bands (∼36 kDa) during HLU compared with non-HLU controls. Densitometry analysis (D) of MSTN bands demonstrated significantly greater intensity (P < 0.05) relative to GAPDH bands in the HLU group compared with non-HLU controls. Equal protein loading of lanes was confirmed by intensity of GAPDH bands.