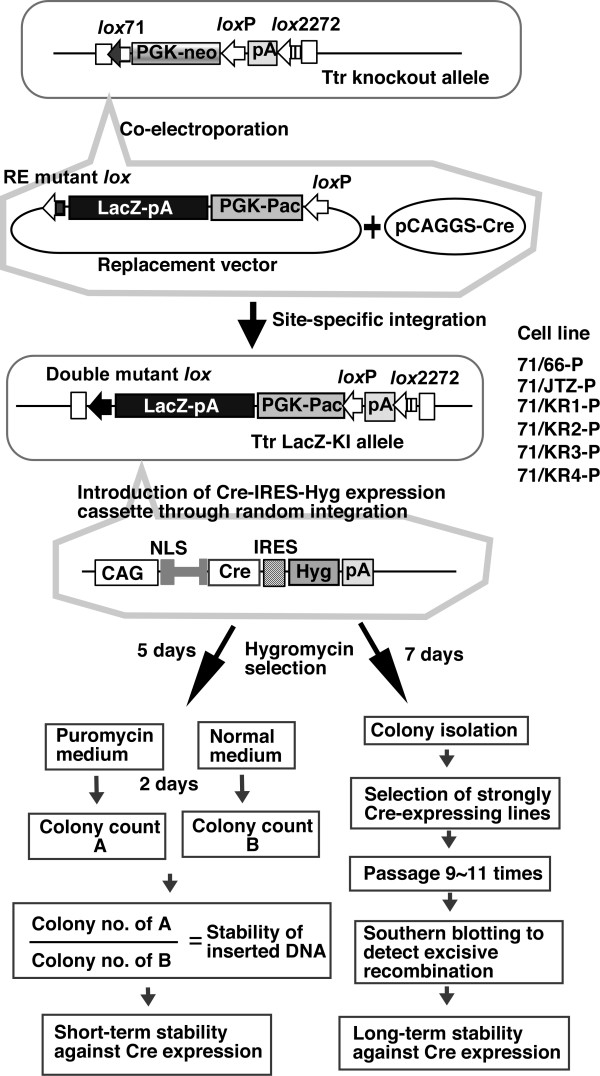
Figure 4.
Strategy to evaluate the stability of double mutant lox. In the Ttr-KO41 line, the lox71-Pgk-neo-loxP-pA-lox2272 cassette was inserted in the first exon of the mouse Ttr gene. Replacement vectors carried the RE mutant lox-LacZ-pA-Pgk-Pac-loxP. The Ttr-KO41 line was coelectroporated with the each replacement vector and the Cre-expressing plasmid; it was then selected with puromycin. Colonies were picked, and six sub-clones (71/66-P, 71/JTZ-P, 71/KR1-P, 71/KR2-P, 71/KR3-P, and 71/KR4-P) carrying different double mutant lox were established. To induce stable Cre-expression, the CAG-Cre-IRES-Hyg transgene was introduced into each sub-clone and selected with hygromycin. For the short-term stability assay, electroporated cells were divided into two plates, and after 5 days of hyg selection, one plate was selected with puro and the other with normal medium. The ratio of hygR puroS colonies to hygR colonies represents the short-term stability of the double mutant lox site. For the long-term stability assay, Cre-expressing sub-lines with high levels were selected and passaged 9~11 times. Genomic DNA was prepared after 4, 7, 9, and 11 passages, and recombination at each stage was detected with Southern blotting.