Table 1. Energetics and structural parameters for the lowest-energy conformers of bradykinin protomers.
| N-ter |
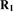
|
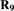
|
C-ter |

|
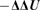
|
 PA PA |
 GB GB |
IR | SB | sHB | iHB | HB |
 = 0 = 0 | ||||||||||||
| 0 | + | 0 | − | 0 | 380 | 409 | 368 | 2 | 1 | 2 | 5 | 1 |
| 0 | 0 | + | − | 10 | 380 | 409 | 368 | 2 | 1 | 2 | 4 | 3 |
| + | 0 | 0 | − | 49 | 542 | 535 | 540 | 2 | 1 | 1 | 5 | 0 |
| 0 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
 = 1+ = 1+ | ||||||||||||
| 0 | + | + | − | 0 | 380 | 409 | 368 | 3 | 2 | 3 | 2 | 0 |
| 0 | + | 0 | 0 | 46 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 4 |
| 0 | 0 | + | 0 | 63 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 1 |
| + | 0 | + | − | 67 | 542 | 535 | 540 | 3 | 2 | 2 | 2 | 3 |
| + | + | 0 | − | 112 | 542 | 535 | 540 | 3 | 1 | 2 | 4 | 0 |
| + | 0 | 0 | 0 | 152 | 162 | 126 | 172 | 1 | 0 | 0 | 3 | 3 |
 = 2+ = 2+ | ||||||||||||
| + | + | + | − | 0 | 542 | 535 | 540 | 4 | 3 | 4 | 3 | 1 |
| 0 | + | + | 0 | 77 | 0 | 0 | 0 | 2 | 0 | 0 | 6 | 1 |
| + | + | 0 | 0 | 85 | 162 | 126 | 172 | 2 | 0 | 0 | 5 | 1 |
| + | 0 | + | 0 | 93 | 162 | 126 | 172 | 2 | 0 | 0 | 5 | 2 |
In each row the following information is reported: protonation pattern (first column); energy difference with respect to the most stable protomer (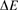 in kJ/mol); (intrinsic) internal energy variation (
in kJ/mol); (intrinsic) internal energy variation (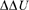 ), proton affinity (
), proton affinity (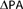 PA) and gas-phase basicity (
PA) and gas-phase basicity ( GPB) relative to the most favourable protomer (see text for a definition of these quantities; all values are in kJ/mol); ionized residues (IR); salt-bridges (SB); hydrogen bonds between salt-bridged residues (sHB); ionized hydrogen bonds where either the donor or the acceptor is ionized,
GPB) relative to the most favourable protomer (see text for a definition of these quantities; all values are in kJ/mol); ionized residues (IR); salt-bridges (SB); hydrogen bonds between salt-bridged residues (sHB); ionized hydrogen bonds where either the donor or the acceptor is ionized, 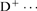 A or D
A or D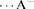 H-bonds (iHB); neutral hydrogen bonds (HB). Hydrogen bonds are identified according to the donor-acceptor (
H-bonds (iHB); neutral hydrogen bonds (HB). Hydrogen bonds are identified according to the donor-acceptor ( ) distance and the donor-acceptor H-bond angle (
) distance and the donor-acceptor H-bond angle (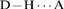 ). The following geometric criterion was adopted:
). The following geometric criterion was adopted:  Å and
Å and  . A salt-bridge is formed if the distance between any oxygen atom of the acidic residue and any protonable nitrogen atom of the basic residue is less than 4.0 Å.
. A salt-bridge is formed if the distance between any oxygen atom of the acidic residue and any protonable nitrogen atom of the basic residue is less than 4.0 Å.
