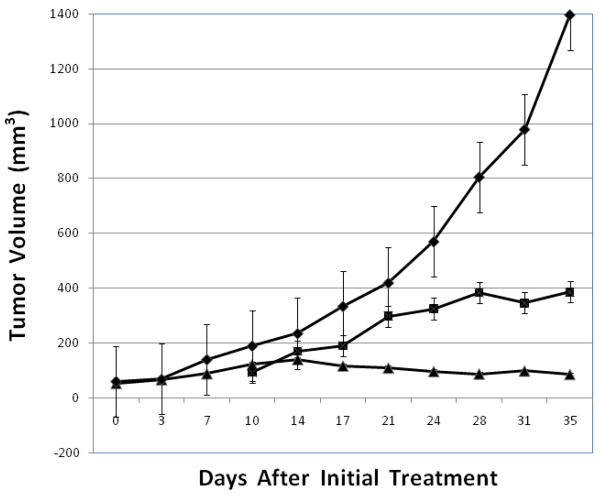
Figure 3.
The test compounds, irinotecan (▲), 10 mM citrate (◆), and 19 (■) were administered by i.p. injection to athymic nude mice with human tumor xenografts established using MDA-MB-435 breast cancer cells. Mice were injected ip 3 × weekly. Negative controls (7 mice) were injected with 150 μl of 10 mM citrate. The positive control group (8 mice) received irinotecan by ip injection at a dose of 20 mg/kg, 3 × weekly, for all four weeks. Compound 19 was similarly administered to seven mice, 3 × weekly, at a dose of 25 mg/kg starting at week 2 with increasing doses of 32 and 42 mg/kg on weeks 3 and 4, respectively. Data are presented as the mean ± SE. The % T/C (average tumor volume of treated as compared to control group) is 27.7% for 19 and 6.1% for irinotecan.