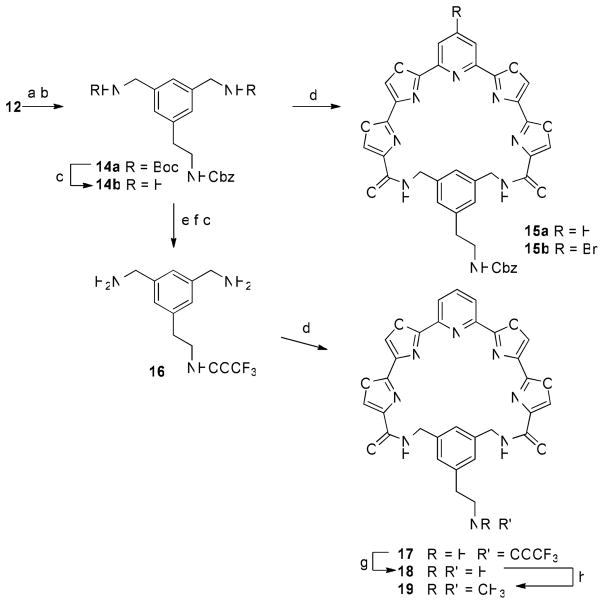
Scheme 5a.
a Reagents and conditions: (a) Boc2O, Et3N, CH2Cl2, rt; (b) CbzNHCH2CH2BF3K, PdCl2(dppf).CH2Cl2, Cs2CO3, toluene/H2O, 80 °C; (c) TFA, CH2Cl2, 0 °C; (d) 5a or 5b (2 mM in DMF), EDC, HOBT, 2,6-lutidine, rt, 2 d; (e) 1 atm H2, 20% Pd(OH)2/C, EtOH, rt; (f) TFAA, pyridine, CH2Cl2, 0 °C; (g) K2CO3, MeOH, Δ; (h) HCHO (aq), NaBH(OAc)3, MeOH/CH2Cl2, rt.