Abstract
Conclusion
A developmental histologic study of the otic capsule indicates that it grows a system of lamellar bone with abundant interconnecting intraosseous channels. These include the “cartilage canals” in the cartilage model, the chondro-osseous and Haversian-like (Volkmann’s) canals in the ossified otic capsule, the fissula ante fenestram, which seems to function as a lifelong manufacturer of the latter two channels, and the inner layer (vestibular arch) of the vestibular aqueduct, which is a complex series of Volkmann’s canals and microcanals. Chemical changes, possibly produced by breakdown of cells within the channels, may provide a homeostatic environment for the functions of hearing and balance that take place in the endolymphatic fluid.
Objectives
We studied the development of the otic capsule in order to clarify the cellular appearances that we had previously described in the normal vestibular arch and the changes in that structure in Méniẻre’s disease.
Method
Step sections from 84 temporal bones, including those from fetuses, children and adults from a variety of ages were examined histologically..
Results
Cartilage canals, bringing blood vessels and mesenchymal cells from perichondrium to the depths of the cartilage model to mediate ossification, are found early in fetal life and disappear when ossification is complete at about 24w. The otic capsule is formed of chondro-osseous canals, which are composed of trabeculae of mineralized cartilage lacunae containing mesenchymal cells that undergo ossification (globuli ossei); also Volkmann’s canals (like Haversian canals in long bones but multidirectional) which are produced from osteoblasts. The lumina of the latter frequently link up with chondro-osseous canals. Lamellar bone forms the background of the otic capsule. The fissula ante fenestram is present from early in the cartilage model and then throughout life. It appears to mediate bone production and the new formation of chondro-osseous channels and Volkmann’s canals. The internal layer of the vestibular aqueduct (vestibular arch) is seen in the cartilage model of the otic capsule (present in early fetal life) as a vascular layer of perichondrally derived connective tissue (not cartilage) surrounding the endolymphatic duct. When endochondral ossification starts, the bone from the adjoining cochlear and vestibular sides embrace this connective tissue layer to form the outer bony layer of the vestibular aqueduct. Osteoblasts then fill the inner layer with lamellar bone and macro and miniVolkmann’s canals. At 1 year osteoblasts in the walls of macroVolkmann’s canals, proliferating thereafter throughout life, produce large numbers of microcanals. It is possible that slow breakdown of these osteoblasts and of similar cells in the canals of the otic capsule proper may contribute to the homeostasis of the endolymphatic duct and that of the the rest of the membranous labyrinth respectively.
Keywords: Endolymphatic duct, microcanals, vestibular arch, Volkmann’s canals, cartilage model, globuli ossei, osteoblasts, homeostasis, development, cartilage canal
Introduction
We have recently observed in sections of normal temporal bones a thin inner skeletal layer of vestibular aqueduct containing numerous small cells, which surrounds the endolymphatic duct throughout most of its length, and forms an arch (the “vestibular arch”) in the vestibular part of its course. In 20 cases of Méniẻre’s disease a degenerative change is observed in those cells, comprising loss of most of them and the apparent new formation of many intraskeletal channels [1].
Our observations suggested that the proximate origin of the hydrops of Méniẻre’s disease could lie in this particular region of the otic capsule. A more precise categorization of the cells and tissues involved seemed necessary to implement further investigation. We felt that this might be achieved by a developmental histological study of the normal otic capsule, including the newly-described vestibular arch, the seat of the main pathological changes.
Pathological changes similar to the skeletal ones that we have described in Méniẻre’s disease have not, to the best of our knowledge, been reported in bones other than the otic capsule. We set out then to scrutinize those aspects of development in the otic capsule which are dissimilar to those of other bones. We soon became aware in this research that intraskeletal channels are very prominent in the development of, and, indeed, also in the mature form of, the otic capsule, which is not so in other bones. Such channels might be relevant to the skeletal changes in Méniẻre’s disease. In this report we will, therefore, focus our description on the channels of the otic capsule.
Material and Methods
In this study, 84 temporal bones were examined. These were from 34 fetuses (comprising 36 temporal bones from 18 whole skulls and 24 isolated temporal bones from 16 fetuses) between 8 and 40 weeks gestation; also from 24 temporal bones from 20 post-partum children and adults of ages spread between 1 and 52 years. Specimens were fixed in 10% formaldehyde and trimmed. Decalcification was carried out by immersion in 10% formic acid. The end point for complete decalcification was determined by radiography. Temporal bones were embedded after dehydration in celloidin. Serial sections were cut at 20–25 μm thickness through the whole temporal bone and one in each 10 sections was stained with hematoxylin and eosin.
Results
The mature otic capsule is composed of three layers of bone – an external layer of periosteal bone, a middle layer of bone formed from cartilage and an inner “endosteal” layer.
Channels of the Middle Layer
Early middle layer otic capsule development is similar to that in long bones. Both commence as cartilage models. Later otic capsule middle layer bone development, however, is profoundly different from that of other bones developed in cartilage. In other bones, cartilage is replaced completely by lamellar bone. In the otic capsule, channel-like structures derived from a chondral framework are formed and maintained throughout life.
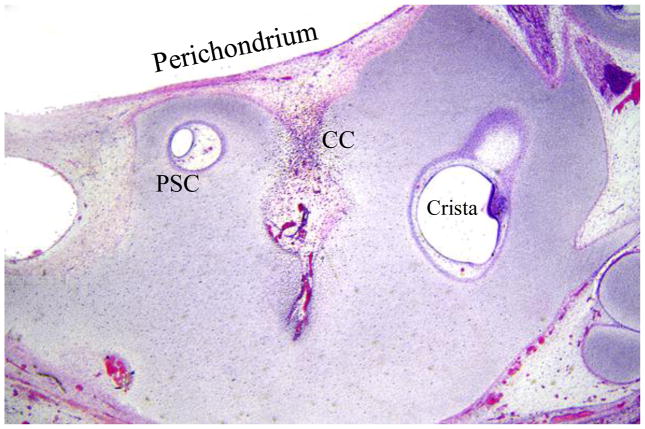
After 8w and until about 16w gestation, when ossification commences, the otic capsule middle layer is composed almost entirely of cartilage. From 11w the vestibular region of the middle layer shows cartilage canals. These structures (which more appropriately should have been termed intracartilage canals rather than the canals derived from cartilage that is suggested by the current terminology) are found in most developing vertebrate cartilages. They bring blood vessels and mesenchymal cells necessary for impending ossification into the depths of cartilage [2] (Figure 1). Ossification takes place along the outer rim of the cartilage canal, after which it disappears.
Figure 1.
Vestibular region of cartilage model of otic capsule at 11 weeks. Note cartilage canal (CC) which brings blood vessels and mesenchymal cells from the perichondrium to a region of the cartilage remote from these structures, eventually to mediate ossification.
PSC: posterior semicircular canal
At about 19w, the cartilage cells destined for subsequent ossification, hypertrophy, undergo apoptotic death and their ground substance becomes mineralized. Mesenchymal cells and small blood vessels enter the empty cartilage lacunae, which are then broken up into spherical masses called globuli ossei. Woven bone fo rms from mesenchymal cells in the globuli ossei. The latter become compressed into trabeculae, separated by large thin-walled blood vessels which later are the site of growth of cellular bone marrow.
Between 20 and 28w the mineralized cartilage framework and the globuli ossei increase in the trabeculae of the otic capsule, which elongate and, together with the thin-walled blood vessels that lie between them, fill an extensive volume of the middle layer of the otic capsule. Spaces appear between the globuli ossei, creating an impression that the chondro-osseous trabeculae of which they are a part, form, in fact, a network of channels.
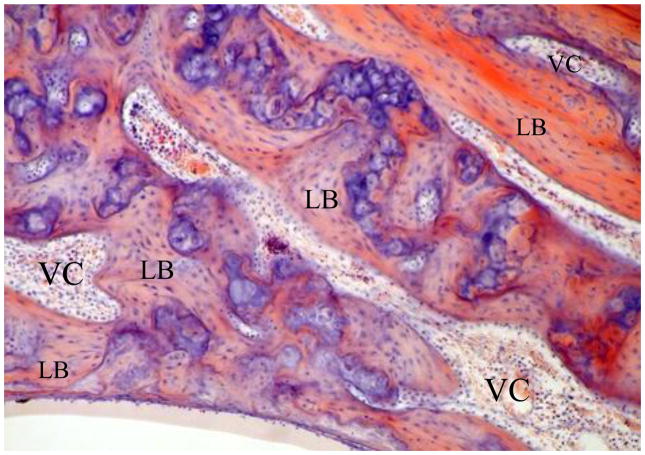
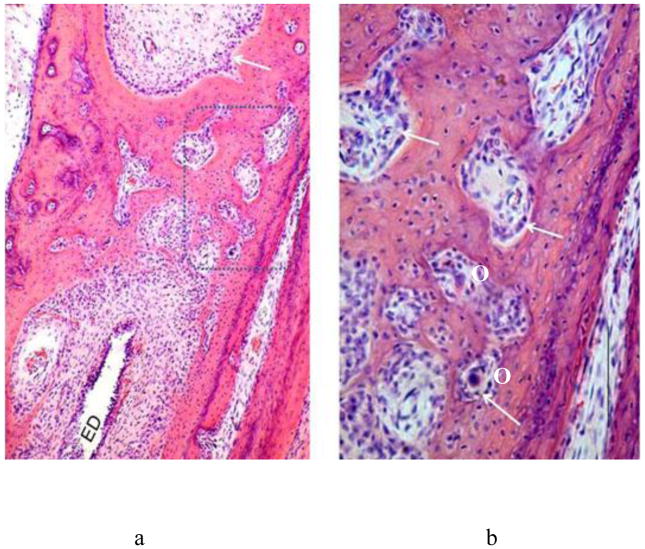
Lamellar bone develops to replace the woven bone in the trabecular channels from an early stage (Figures 2 and 3). This mature bone is formed by a single layer of osteoblasts on their inner surfaces and these cells secrete layer after layer of bone on this surface over the chondro-osseous trabecular channels. The expanding plaques of lamellar bone push out the osteoblasts which eventually embrace on their outer surfaces blood vessels, mesenchymal cells and, in some places, even nerves. Such lamellar bone expansion does not reach its mature stage of closing down to become thin channels until about the first postpartum year. In their mode of development, structure, basophilic pigmentation, contents and relationship to the surrounding lamellar bone these thin channels closely resemble the Haversian canals of other bones. Unlike the latter, they are very numerous throughout the whole otic capsule, and run in all directions, in contrast to the strictly right-angled directionality of the Haversian canals in long bones. It would seem reasonable, therefore, that the abundant channels of the otic capsule should be termed Volkmann’s canals, the name given to the multidirectional channels of other bones (Figure 3). In many places lumina of Volkmann’s canals fuse with globuli ossei of chondro-osseous canals. The similarity between the Volkmann’s canals of the otic capsule and the Haversian canals of other bones extends to the observation that each forms a central focus for a concentrically laminated system of lamellar bone. In the temporal bone, however, the deposition of such systems is irregularly patterned unlike the strictly geometrical deposition of “osteons”, as such arrangements are termed in the long bones. Volkmann’s canals in the otic capsule, like Haversian canals in long bones, also form a focus for a series of very fine channels, which radiate among the fibers of the lamellar bone - the well-described canaliculi.
Figure 2.
Chondro-osseous channels with globuli ossei (stained blue) at 25 weeks in an enlarging cartilage framework and early Volkmann’s canals (VC) forming from lamellar bone (LB).
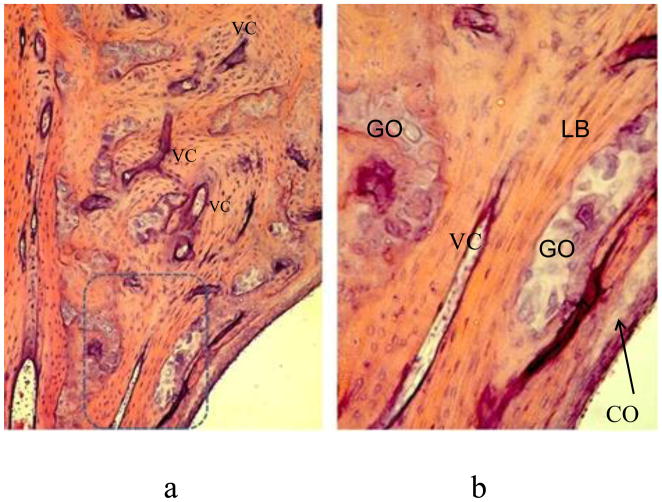
Figure 3.
a: Middle layer of otic capsule at 2 years. Lamellar bone proliferation has tightened up Volkmann’s canals, some with their enclosed blood vessels and mesenchymal cells. b: Enlarged view of box in a. Note globuli ossei in internal layer.
GO: globuli ossei in chondro-osseous canal. LB: Lamellar bone. VC: Volkmann’s canal. Arrow: Globuli ossei in inner layer of otic capsule at junction with cochlea (CO).
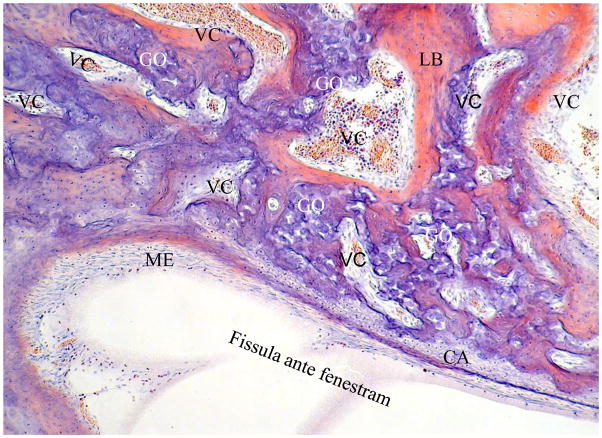
A cleft in the cartilage model of the otic capsule – the fissula ante fenestram, reaching from the vestibule near the footplate up towards the basal coil of the cochlea, and containing mesenchymal cells and blood vessels, can be observed from as early as 11w and is well-formed at 16w. It is later, throughout life in most temporal bones, seen as the hub of condensed and differentiating bone and channel-forming activity, i.e. it is a life-long cartilage canal. At 23w its vestibular end is filled with mesenchymal cells, while towards the cochlea a large mass of cartilage survives until late in childhood. Around the mesenchymal cells there is a thin layer of hyaline cartilage. Early chondro-osseous channels form from the latter with large numbers of globuli ossei fragmenting from the mineralised, mesenchymal cell-occupied cartilage and becoming arranged into trabeculae. They are accompanied by differentiating Volkmann’s canals which are being formed around the fissular edges by the folding of lamellar bone around blood vessels and groups of mesenchymal cells (Figure 4). The mature fissula appears to mediate growth directly of both narrow Volkmann’s canals and chondro-osseous channels with globuli ossei from one year and thereafter throughout life.
Figure 4.
Fissula ante fenestram at 39 weeks. Volkmann’s canals (VC) are being formed from the fissula edges by the folding of lamellar bone over blood vessels and groups of mesenchymal cells.
CA: residual cartilage layer of fissula from which globuli ossei are derived. GO: globuli ossei in chondro-osseous canals also formed from fissula. LB: lamellar bone. ME: mesenchymal and blood vessels of inner cellular layer of fissula.
Channels of the External Periosteal Layer
On the surface of the cartilage layer in the cartilage model of the otic capsule lies a thin perichondral/periosteal layer of mesenchymal cells. At about 20w this external layer starts to ossify, but cartilage does not play a part in this process. A thin sheet of woven bone is formed directly from mesenchymal cells between the spindle cells of the external layer. By 23w this has thickened and become largely lamellar bone. Small blood vessels and osteoblasts are formed concomitantly and the deposition of lamellar bone around groups of them gives rise to early Volkmann’s canals. By 28w the external layer of outer periosteal bone with numerous Volkmann’s canals has become thick around the cochlea, except in the fissular region where the external layer is thin. A moderately thick layer of the same bony tissue with Volkmann’s canals is seen around the vestibular region. The same relative amounts of external layer bony tissue are in place throughout life.
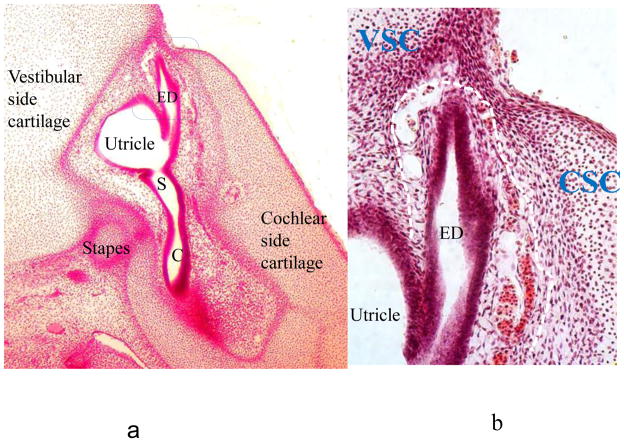
The bony vestibular arch is the inner layer of the vestibular aqueduct, termed as an arch because of its configuration in the posterior vestibule [1]. In early fetal life the differentiated inner ear membranous structures have become surrounded by a cartilaginous model of the otic capsule in the region of its cochlear and vestibular portions. The cartilage is covered by a perichondral connective tissue layer. At 8 weeks development the endolymphatic duct/sac portion of that labyrinth has its own connective tissue covering which is very vascular. This is the primordium of the vestibular arch. The cartilage and its perichondrium adjacent to the endolymphatic duct on both its cochlear (medial) and vestibular (lateral) sides are seen to have grown up alongside the endolymphatic duct and embraced it closely. The cartilage from both sides will become the outer layer of the vestibular aqueduct (Figure 5).
Figure 5.
a: low power view of primordial otic capsule at 8w gestation.
C: cochlea. ED: endolymphatic duct. S: saccule.
b: high power view of otic capsule at 8w (same section as a) in the region of the endolymphatic duct. The vascular primordial vestibular arch (connective tissue layer of endolymphatic duct) is outlined by a white dashed line.
CSC: cochlear side cartilage. ED: endolymphatic duct. VSC: vestibular side cartilage.
At about 20 weeks, in the region where the endolymphatic duct/sac passes posterolaterally to a subdural position from between the cochlear and vestibular portions of the otic capsule, the cartilage model of the latter two zones starts to ossify and the anterior spread of this endochondral ossification leads to the formation of the outer layer of the vestibular aqueduct. A thin band of woven bone forms from the vestibular arch connective tissue at the interface with the endochondral bone all around it and a sharp-pointed extension of this woven bone grows anteriorly to separate the arch tissue from the utricle (Figure 6). Woven bone also grows as a bridge around the distal portion of the endolymphatic duct near its transition to the wider endolymphatic sac and expands replacing connective tissue towards the vestibule. The separate origins of the osteogenic vestibular arch bone (inner vestibular aqueduct layer) on the one hand and the surrounding endochondrally derived bone (outer vestibular aqueduct layer) on the other, are clearly shown histologically from this time and throughout life, by a thin blue line (tidemark) denoting the edge of endochondral ossification, and also by the presence of numerous zones of globuli ossei in the outer (endochondrally-derived bone), but not in the inner (osteogenic vestibular arch bone) regions (Figure 7).
Figure 6.
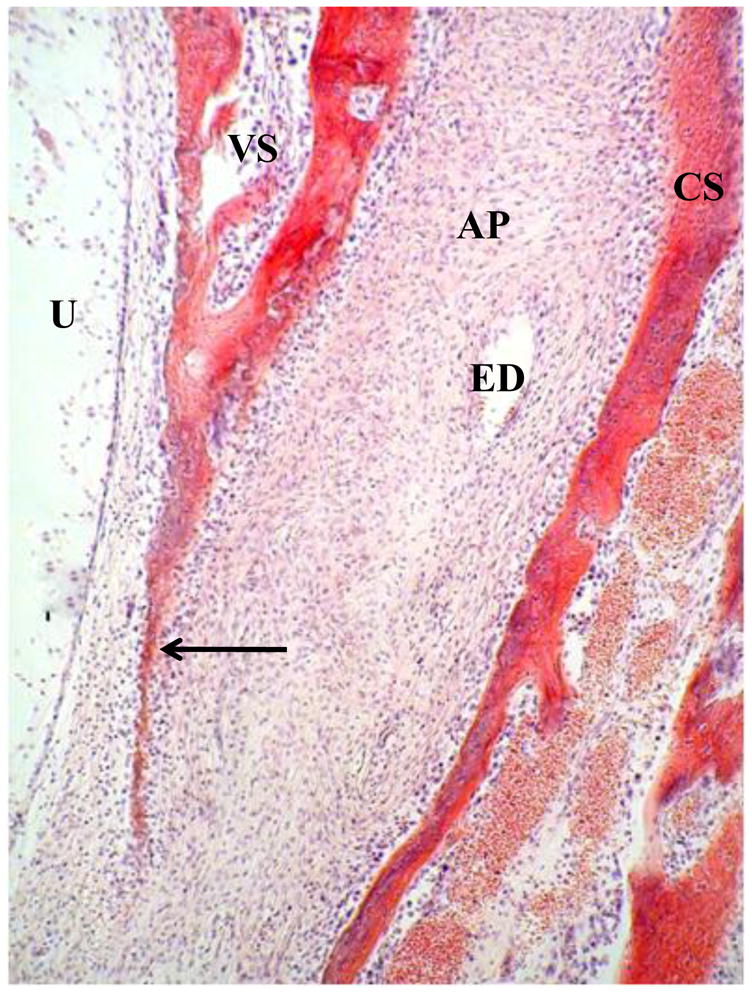
Anterior region of vestibular arch at 20w. AP: connective tissue primordium of vestibular arch. CS: cochlear side of otic capsule. U: Utricle. VS: vestibular side of otic capsule. Arrow: sharp-pointed extension of woven bone (future lateral band of vestibular arch) separating arch tissue from utricle.
Figure 7.
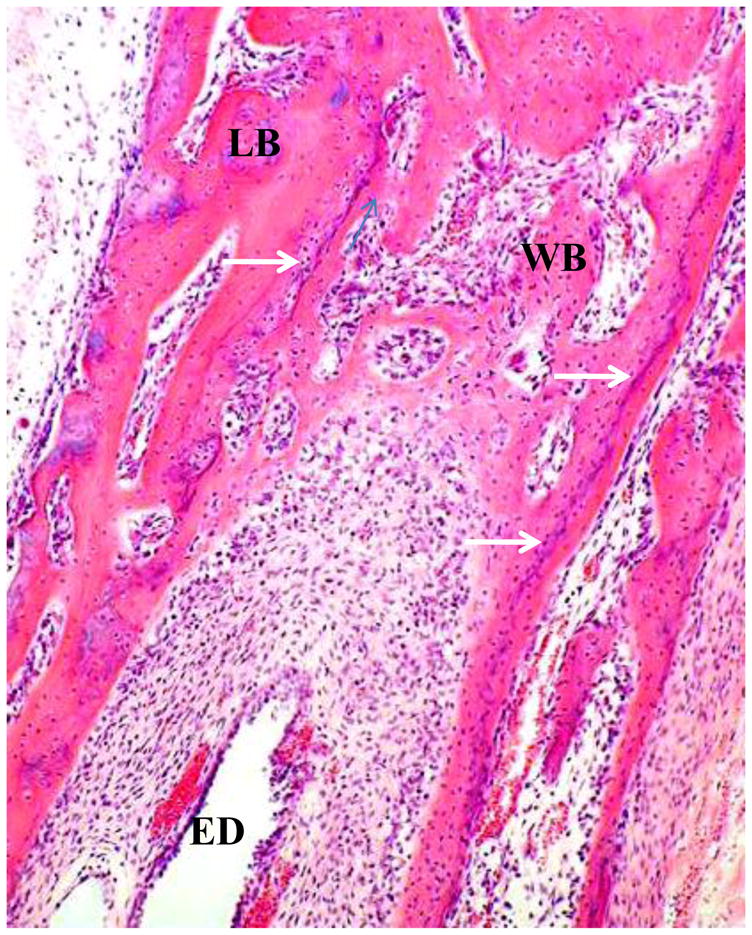
Vestibular arch at 24w. WB: woven bone of vestibular arch. ED: endolymphatic duct. LB: lamellar endochondral bone (outer layer of vestibular aqueduct). White arrows: tidemarks showing interface between endochondral ossification and ossification from membrane.
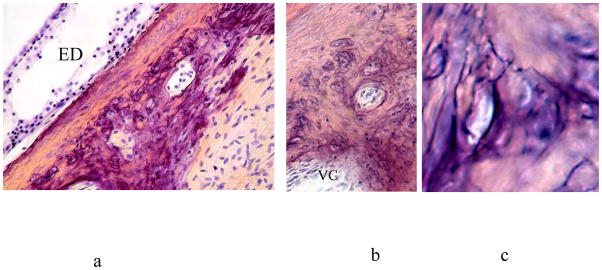
Like the rest of external bony covering of the otic capsule, from which it has a similar origin, the skeleton of the vestibular arch starts to form lamellar bone from woven bone at about 24w, with osteoblasts surrounding connective tissue cells and vascular spaces, producing incipient Volkmann’s canals (Figure 8). The subsequent development of the vestibular arch from full term onwards is characterized by the formation of many Volkmann’s canals, ranging from major ones, bifurcating to produce a medial (cochlear capsular side) canal and a lateral (vestibular capsular side) canal, to small ones. Some Volkmann’s canals show thin-walled blood vessels within their lumina. From about 40 weeks gestation, a miniature form of canalization begins to take place. These microcanals begin on the arch near the endolymphatic duct as narrow layers of lamellar bone. These layers are elongated in a direction parallel to that of the nearby epithelium of the endolymphatic duct and are accompanied by many osteoblasts. At one year post-partum, prominent osteoblasts in the apex of the arch surround and cover openings into superficial pits which have formed in the layered bony material. This seems to be an early stage of a microcanalization process in which the bone in the region of the arch surrounding the endolymphatic duct is invaded by fine channels. By two years and thereafter throughout life this is seen in the apex of the arch as small Volkmann’s canals and microcanals which are so small as to be seen only at very high power of the light microscope. Prominent osteoblasts also cover the whole surface of this microcanalizing area (Figure 9). Because of the irregular density in three dimensions that it produces within the 20μm thick sections, these changes give a blurry, basophilic appearance to the arch region.
Figure 8.
a Low power. b: Higher power of dotted rectangular area in a. Vestibular arch at 28w. Volkmanns canals, delineated by some rows of surface osteoblasts (white arrows) have formed in the lamellar bone. ED: endolymphatic duct. O: osteoclasts.
Figure 9.
Apex of vestibular arch in a child of six years. a and b: small Volkmann’s canals amid prominent osteoblasts. The corner of a larger Volkmann’s canal (VC) is seen at bottom left in b. c: Very high power. Minute canals, revealed by their cut surfaces and also by their contour in the one with an intact surface, are also present in the region, accompanied by osteoblasts in poor focus at this power. ED: Endolymphatic duct.
The Internal Layer
At 23w trabeculae of globuli ossei are present throughout the otic capsule and form a layer of globuli ossei at the junction of the otic capsule with membranous labyrinth. Later in fetal life they appear to be fused together to form a thin amorphous band, although some globuli ossei may be seen here in later life at any age (Figure 3). Groups of globuli ossei may also be seen in that layer at the junction with the spiral ligament throughout life. Lamellar bone is produced on the external side of the internal layer, but actual ossification of this layer does not occur.
Discussion
Most of the structures seen in the development of the otic capsule can be identified in the development of other bones of the body. Our study of the development of the otic capsule, however, indicates a marked dissimilarity between the detailed construction of this tissue and that of other bones. The persistence in the middle layer throughout life of a cartilage-based array of canals which anastomose with Haversian-like canals and the large numbers and diversity of the latter in all parts of the otic capsule indicate that it is likely to be a tubular system primarily formed for the purpose of the handling and transmission of fluids. We have generated new observations in respect of five types of channel which are prominent in this bone (Table 1):
Table 1.
Channels of Otic Capsule
| Appear | Disappear | Site | Function | |
|---|---|---|---|---|
| Cartilage Canals | 11w | 21w | Vestibular part of cartilage model | Mediate ossification of cartilage model |
| Chondro-osseous canals | 21w | _ | Middle layer otic capsule | ?Homeostasis |
| Volkmann’s Canals | 28w | _ | All regions of otic? capsule | ?Homeostasis |
| Fissula ante fenestram | 11w | _ | Near cochlea to vestibule | Mediates ossification throughout life |
| Vestibular arch: Volkmann’s canals and microcanalization | Before 8w | _ | Inner layer vestibular aqueduct and vestibule | ?Homeostasis |
Cartilage canals formed in the vestibular capsule cartilage model, temporarily provide the blood vessels and stem cells necessary to mediate ossification.
The mineralized cartilage trabecular framework formed early in ossification is not resorbed. Its lacunae are filled with mesenchymal cells soon ossified (globuli ossei). Elongated groups of this material with a covering of woven bone, followed by lamellar bone, fill much of the middle layer of the otic capsule (chondro-osseous trabeculae). These structures have been thought by many to be just fetal remnants [3], a concept supported on the basis of the discovery that remodeling of bone does not occur in the otic capsule. Thus ossification cannot proceed to the osteonal remodeling found in long bones and perforce leaves behind these unused remains of development. We find difficulty in accepting this concept and believe rather that they may be channels functioning throughout life for the following reasons: (a) unused fetal remnants usually degenerate and disappear during early childhood, whereas these structures are prominent features of the otic capsule in all mature temporal bones. Indeed, observation of the skeletal tissue around the fissula ante fenestram suggests that this is a source of newly-formed chondro-osseous channels throughout life (see below) (b) chondro-osseous trabeculae develop spaces, so-called “interglobular spaces”, within the trabeculae, that may be seen throughout life, suggesting possible regions through which soluble substances can pass. (c) chondro-osseous channels show direct communication with Volkmann’s canals in many places. The latter are definite channels suggesting that the former may also serve the same function.
Osteoblasts repeatedly deposit layers of lamellar bone on the surfaces of chondro-osseous channels (trabeculae) with globuli ossei and enclose blood vessels and mesenchymal cells. These enclosures (Volkmann’s canals), are multidirectional forms of Haversian canals and, although well-described in long bones [4], and prominent in the otic capsule, have received little attention in the literature. Volkmann’s canals form an extensive network in both the external layer of the otic capsule and, in the middle layer, where they anastomose freely with chondro-osseous canals with globuli ossei, their prominence is even greater.
Homeostasis of the endolymph fluid environment, where sound and balance sensory reception takes place, is important. A powerful chemical activity is required to produce the unique chemical composition of the endolymph, with its very high potassium and very low sodium levels. The endocochlear potential must be the result of powerful and complex chemical changes. Endolymph activities involving protein, purine even hormonal changes have been detected. The source of some of these chemical alterations have been localised to the stria vascularis and the spiral ligament within the cochlea, but few vestibular structures have been suspected to have such a role. The presence of a large system of channels in the skeletal tissue immediately surrounding the membranous labyrinth and separated from it by only a thin membrane composed largely of compressed globuli ossei, suggests to us that this system may function to provide some of the basic mechanisms for such homeostatic activities. Certainly, a copious vascular system is available within the Volkmann’s canals, with protection from damage of the delicate vascular structures by the surrounding lamellar bone. It is possible that the chondro-osseous channels, also, may be the source of some specialised chemical activities, their cartilaginous structure allowing free diffusion of soluble substances towards and into the membranous labyrinth, but, the surrounding bone, at the same time preventing diffusion of those substances away from those channels..
The fissula ante fenestram opens into the vestibule, but we have shown that in all cases it burrows deeply medially and superiorly into the cochlear part of the otic capsule. The anatomy of this structure has, in fact, been well described [5], but no function has as yet been ascribed to it. In the present study we have confirmed that the fissula is a constant feature in human temporal bones from early in the cartilage model phase and subsequently throughout life. After ossification of the otic capsule, the latter is widened in the region of the fissula with a thin covering outer periosteal bony layer in contrast to the thick periosteal layer around the rest of the otic capsule. The bone around the fissula is always packed densely with newly formed cartilage and new bone and so continually appears to mediate the formation of chondro-osseous channels with globuli ossei and Volkmann’s canals and this process seems to continue throughout life. To the best of our knowledge there has been no previous suggestion in the literature that this structure could act in this way as a permanent “cartilage canal”, i.e. an organ for the production of new bony structures.
In an X-ray study of undecalcified sections of the temporal bone of a patient who had received tetracycline treatment for infection before death, Sǿrensen et al. [3] noted new bone formation, revealed as an opacity in the wall of the fissula ante fenestram, but the possibility that this structure could act to mediate bone production was not discussed. It is well known that the fissula ante fenestram frequently seems to be the source of new otosclerotic bone.
When we made our original description of the vestibular arch [1], we had not studied the developmental details of this skeletal structure or those of the otic capsule as a whole of which it is a part. It is now necessary to revise some of the histological interpretations that we put forward in that study in the light of our new developmental findings of this part.
We were not aware when we wrote that paper that the vestibular arch, like much of the otic capsule, has, as we have now shown here, a foundation of lamellar bone. We indicated also in that paper that “atypical blood vessels” were numerous in the bone of the arch. It now appears clear that the majority of the large and small channels there are, in fact, Volkmann’s canals, which often contain blood vessels, but are not themselves blood vessels. Thus the “blood vessels” mentioned in the captions of Figs. 2 and 3 of our 2009 paper [1] are, in fact, Volkmann’s canals. We were also not aware of the presence of micro Volkmann’s canals and microcanals, the development of which is newly described in the present work.
The intraosseous cells with accentuated cell walls that are numerous in mature vestibular arches were interpreted as “mineralized chondrocytes” in the previous publication. They can now be interpreted more probably as osteoblasts, i.e. bone cells with a mineralized edge. Normal cartilage is not seen at any stage of development of the vestibular arch. We now know, in fact, that the arch is not derived from the cartilage model, but, rather, is formed entirely from the osteogenic cells of the external layer of the otic capsule.
Osteoblasts are the predominant, active cells of lamellar bone and by their activity are responsible for the formation of Volkmann’s canals and probably the microchannels of the arch. The process of ossicular microcanalization commences very near to the epithelial lining of the endolymphatic duct and, indeed, this may be programed to take place by the very proximity of that epithelium to the osteoblasts of that region. The development of the otic capsule skeleton is known to be activated early by signaling molecules from the nearby primordial epithelium, including that of the endolymphatic duct [6]. The continuance throughout life of such a relationship between endolymphatic duct epithelium and the nearby vestibular arch tissue may result in osteoblast proliferation and consequent microcanalization.
Slow breakdown of the proliferated osteoblasts by apoptosis, as we suggested to happen in the previous publication [1] may serve to nourish the nearby endolymph, posssibly with regard to substances such as potassium ions, in a homeostatic function similar to that which we suggested above to be a possible function of the abundant chondro-osseous channels and Volkmann’s canals of the middle layer of the otic capsule in relation to the cochlear and vestibular parts of the membranous labyrinth.
As we described above, the process of microcanalization starts at about the time of birth. This is when the vestibular-regulated activities of head motion become important to the newborn baby. The process of microcanalization matures by one to two years of age, when fine tuning of the vestibular endolymph-based reflexes is required for walking. Thus increasing elaboration of progressively finer canals seems to be chronologically matched with the need for more delicate homeostasis in the vestibular fluid environment. The endolymphatic duct is strategically placed in relation to the sensory structures important in balance, the saccule and utricle, as regard its propinquity to and continuity of a lumen with them, so that changes in endolymph composition induced in the vestibular arch region could easily be transmitted by diffusion to those parts.
Acknowledgments
We would like to t hank Robert Chapman, of the Department of Histopathology, Charing Cross Hospital Campus, Imperial College Healthcare NHS Trust, London and Daniel Jagger PhD, of the UCL Ear Institute, London for their expert help with the photography for this publication.
Footnotes
This work was first presented at the 32nd Annual Midwinter Meeting of the Association for Research in Otolaryngology, Baltimore MD, February 2009
References
- 1.Michaels L, Soucek S, Linthicum F. The intravestibular source of the vestibular aqueduct: its structure and pathology in Ménière’s disease. Acta Oto-Laryngologica. 2009;129:592–601. doi: 10.1080/00016480802342416. [DOI] [PubMed] [Google Scholar]
- 2.Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat. 2008;190:305–315. doi: 10.1016/j.aanat.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Sǿrensen MS, Bretlau P, Jǿrgensen MB. Bone remodeling in the otic capsule. Some morphological findings. Acta Otolaryngol (Stockh) 1992;(Suppl 496):11–19. [Google Scholar]
- 4.Pritchard JI. Chapter 1. General histology of bone. In: Bourne GH, editor. The biochemistry and physiology of bone. Vol. 1. Academic Press; New York: 1972. p. 14. [Google Scholar]
- 5.Bast TH, Anson BJ. The temporal bone and the ear. Charles C Thomas; Springfield, Illinois, USA: 1949. [Google Scholar]
- 6.Liu W, Ha Oh S, koo Kang Y, Li G, Doan TM, Little M, et al. Bone morphogenetic protein 4 (BMP4): a regulator of capsule chondrogenesis in the developing mouse inner ear. Developmental Dynamics. 2003;226:427–438. doi: 10.1002/dvdy.10258. [DOI] [PubMed] [Google Scholar]