Figure 4.
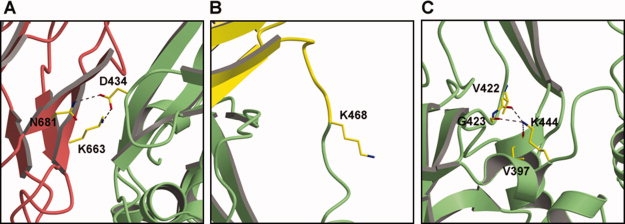
Structural context of the three Lys residues in TGM2 that are acetylated by ASA. (A) Lys663 hydrogen bonds with Asp434 across the β2-core domain interface in the closed form of TGM2. This interaction is absent in the open form [see Fig. 3(B)]. (B) Lys468 is located within a disordered loop of TGM2 that connects the core and β1 domains. Although Lys468 was included in the crystal structure of the closed form of TGM2, the electron density is weak or absent in all molecules of the asymmetric unit, indicating its disorder. (C) Lys444 is relatively buried within the core domain where it forms hydrogen bonds with three main chain carbonyls. Shown here is the closed form of TGM2, but the structure is essentially the same in the open form. For all three panels, the views used are approximately the same as in Figure 3 and the same colors are used for the domains.
