Figure 2.
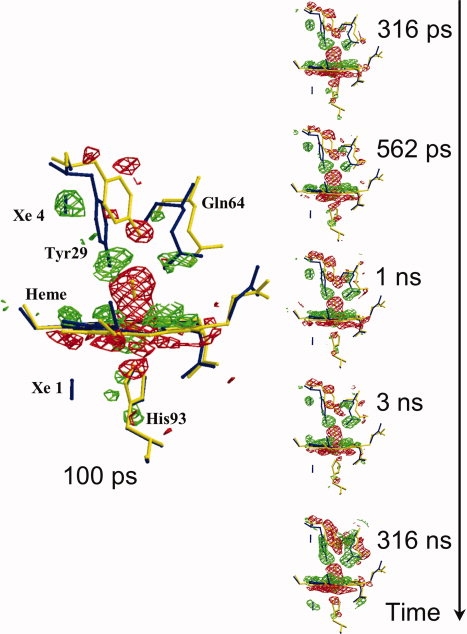
Structural changes in the heme vicinity for the mutant called YQR-Mb,43 from 100 ps to 316 ns after CO photolysis. YQR-Mb is a triple mutant (L29Y/H64Q/T67R) used for this experimental session because of favorable properties (zero CO geminate recombination, slow bimolecular rebinding, and excellent sturdy crystals). (Flight − Fdark), the difference electron density maps, are in red for negative and in green for positive (contoured at 3.0 σ); these are overlaid on models of YQR-MbCO (in yellow) and YQR-Mb (in blue). It may be seen that at 100 ps, the negative density (red) of the Fe2+CO on the distal side of the heme is very prominent; other detectable changes are the initial distortion of the heme and motions of the distal Tyr29 and Gln64. These structural changes continue to grow with time and are fully developed in the frame at 316 ns. In all the frames, a blue bar indicates the position of photolyzed CO migrating initially to the Xe4 pocket (distal, at the top in the 100 ps frame) and later to the Xe1 pocket (proximal, at the bottom, better seen in the frame at 316 ns) (from Bourgeois et al.,44 modified).
