Abstract
Endothelial cell death due to increased reactive oxygen species (ROS) may contribute to the initial endothelial injury, which promotes atherosclerotic lesion formation. Piper sarmentosum (PS), a natural product, has been shown to have an antioxidant property, which is hypothesized to inhibit production of ROS and prevent cell injury. Thus, the present study was designed to determine the effects of PS on the hydrogen peroxide (H2O2)-induced oxidative cell damage in cultured human umbilical vein endothelial cells (HUVECs). In this experiment, HUVECs were obtained by collagenase perfusion of the large vein in the umbilical cord and cultured in medium M200 supplemented with low serum growth supplementation (LSGS). HUVECs were treated with various concentrations of H2O2 (0–1000 µmol/L) and it was observed that 180 µmol/L H2O2 reduced cell viability by 50% as denoted by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Using the above concentration as the positive control, the H2O2-induced HUVECs were concomitantly treated with various concentrations (100, 150, 250 and 300 µg/ml) of three different extracts (aqueous, methanol and hexane) of PS. Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) levels showed a significant increase (P<0.05) in HUVECs compared to the negative control. However, PS extracts showed a protective effect on HUVECs from H2O2-induced cell apoptosis with a significant reduction in MDA, SOD, CAT and GPX levels (P<0.05). Furthermore, PS had exhibited ferric reducing antioxidant power with its high phenolic content. Hence, it was concluded that PS plays a beneficial role in reducing oxidative stress in H2O2-induced HUVECs.
Keywords: Piper sarmentosum, Human umbilical vein endothelial cells (HUVECs), Malondialdehyde, Oxidative stress, Antioxidant enzymes
1. Introduction
Piper sarmentosum (PS) is widely found to inhabit the tropical and subtropical regions of the world. It has been used as an expectorant in Thailand (Pongboonrod, 1976). Its leaves and roots have been reported to be used for treatment of toothache, fungoid dermatitis, asthma, and pleurisy (Perry, 1981). Previous investigations highlighted the hypoglycemic action of the PS extract in rats and in alloxan-treated rabbits (Pongmarutai, 1980; Peungvicha et al., 1998). In Malaysia, this plant has been traditionally used to treat hypertension and diabetes mellitus. The methanolic extract of PS leaves has been reported to exhibit antioxidative activity by virtue of possessing the natural antioxidant superoxide scavenger, naringenin (Subramaniam et al., 2003; Chanwitheesuk et al., 2005).
Oxidative stress plays an important role in the pathogenesis of various cardiovascular diseases including atherosclerosis (Ross, 1999). Oxidative stress results from the imbalance between the prooxidant and the antioxidative defense mechanisms of the body. At high concentrations, reactive oxygen species (ROS) can cause severe damage to cellular structures and components including nucleic acids, proteins, and lipids, thereby leading to cell death. Malondialdehyde (MDA) is the most abundant product of polyunsaturated lipid peroxidation (Del Rio et al., 2005). The level of MDA has been investigated extensively as an indicator of oxidative damage by determination of its derivation with thiobarbituric acid.
The endothelial cells that line the blood vessels are very sensitive to injury caused by oxidative stress. Endothelial cells play an important role in physiologic hemostasis, blood vessel permeability, and response of blood vessel to other physiologic and pathologic stimuli. Any abnormality in endothelial cell structure and function may contribute significantly to the blood vessel diseases such as thrombosis, atherosclerosis, and vasculitis. Past research studies have used HUVECs for in vitro experiments related to vascular dysfunction (Jaffe et al., 1973).
The antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) and the antioxidants like vitamin C, vitamin E, glutathione, carotenoids, flavonoids, and micronutrient elements offer protection against oxidative damage. There is an increased trend worldwide to use natural antioxidants derived from plant materials such as tea, vegetables, herbs, oilseeds, beans, and fruits as an alternative intervention against oxidative stress-related diseases. An extensive search of literature depicts that there are few studies on the antioxidant effects of PS in cardiovascular diseases. This prompted us to embark on the study to determine the antioxidant role of PS as a nutritional countermeasure to attenuate oxidative stress in cardiovascular dysfunction. Specifically, the hypothesis is that PS would reduce the MDA, SOD, CAT, and GPX levels as oxidative stress markers in H2O2-induced HUVECs.
2. Materials and methods
2.1. Materials
Medium M200 and low serum growth supplementation (LSGS) kits were purchased from Cascade Biologics, USA. Collagenase enzyme Type 1 was obtained from Worthington Biochemical Corporation, USA. Trypsin-ethylenediaminetetraacetic acid (EDTA) and penicillin and streptomycin antibiotics were obtained from Invitrogen, USA. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulphoxide (DMSO), 2,4,6-tripyridyl-S-triazine (TPTZ), Folin-Ciocalteu reagent, ferric chloride (FeCl3middot;6H2O), ferrous sulphate (FeSO4·7H2O), trichloroacetic acid (TCA), thiobarbituric acid (TBA), hydrochloric acid (HCl), butylhydroxytoluene (BHT), 1,1,3,3-tetraethoxypropane (TEP), potassium dihydrogen phosphate, disodium hydrogen phosphate, EDTA, L-methionine, nitroblue tetrazolium (NBT), riboflavin, natrium azide, nicotinamide adenine dinucleotide phosphate (NADPH), glutathione reductase (GSSG), glutathione (GSH), Triton X-100, sucrose, and hydrogen peroxide were purchased from Sigma-Aldrich Co., USA. All other chemicals used are of analytical grade.
2.2. Preparation of Piper sarmentosum extract
Fresh leaves (3 kg) of PS were collected from the Ethnobotanical Garden, Forest Research Institute Malaysia (FRIM), after being identified and confirmed by a plant taxonomist from the Medicinal Plant Division (voucher specimen, FRI 45870), FRIM. All the extraction procedures were performed at the FRIM laboratory. Slices of dry leaves of PS were extracted by aqueous, methanol, and hexane at 80 °C for 3 h respectively (10%, w/v). The methanol and hexane extracts were concentrated with a vacuum rotary evaporator under reduced pressure while the aqueous extract was freeze-dried to obtain the crude PS. All the extracts were stored at 4 °C until further use.
2.3. Ferric-reducing antioxidant power activity
The total antioxidant potential of PS extract was determined using the ferric-reducing antioxidant power (FRAP) assay (Benzie and Strain, 1996). This assay is based on the reduction of Fe3+-TPTZ to a blue-colored Fe2+-TPTZ. The absorbance was read at 593 nm spectrophotometrically (Shimadzu, Japan). The FRAP value in sample was determined from a standard curve plotted using ferrous sulphate and the result was stated in the unit of µmol Fe(II) per gram dry matter (DM).
2.4. Total phenolics content
The total phenolics content (TPC) was determined using Folin-Ciocalteu reagent method (Velioglu et al., 1998). The absorbance was measured at 725 nm spectrophotometrically (Shimadzu, Japan) and the result was expressed as milligrams of gallic acid equivalent (GAE) per gram dry matter (DM).
2.5. Endothelial cell isolation and culture
Human umbilical cords were obtained from healthy women who underwent uncomplicated term pregnancies. Informed consent was obtained from each subject and the present study was approved by the Ethical Research Committee of Universiti Kebangsaan Malaysia Medical Centre (FF-138-2007). HUVECs were isolated from human umbilical cord veins by using an enzymatic technique with slight alteration in the described procedure (Jaffe et al., 1973). Briefly, the veins were perfused with cord buffer, then infused with 0.1% (w/v) collagenase, clamped at both ends, and incubated at 37 °C for 15 min. Then, the collagenase solution was decanted into a plastic centrifuge tube and centrifuged at 1200 r/min for 10 min. The cell pellet was resuspended in medium M200 supplemented with LSGS. The cells were grown to confluence at 37 °C in a humidified 5% CO2 incubator on tissue culture flasks. The medium was replaced twice a week and passaged every three to four days at a ratio of 1:3. Cells were used up to the fifth passage for all experiments. HUVECs were identified by the typical cobblestone morphology and immunofluorescence staining by monoclonal antibodies against von Willebrand factor (Affinity Biologicals Inc., USA).
2.6. Measurement of cell viability
The assay was performed by seeding HUVECs at concentration of 5×104 cells/well in 96-well plates. The cells were treated with various concentrations of H2O2 (0 to 1000 µmol/L) or various concentrations of three different extracts of PS (aqueous, methanol, and hexane) (0 to 1000 µg/ml) respectively for 24 or 72 h. In order to examine the treatment effect of PS on H2O2-oxidative cell damage, the cells were exposed to H2O2 (180 µmol/L) and concomitantly treated with three different extracts (aqueous, methanol, and hexane) of PS (0 to 1000 µg/ml) for 24 h. Cell viability was assessed by MTT assay (Takahashi et al., 2002). The absorbance of each well was immediately measured at 570 nm with enzyme-linked immunosorbent assay (ELISA) microplate reader (Versamax, USA).
2.7. Experimental protocol
HUVECs were plated onto 6-well tissue culture plates and allowed to attach for a 24 h period. Cells were exposed to 180 µmol/L hydrogen peroxide and concomitantly treated with various concentrations (100, 150, 250 and 300 µg/ml) of three different extracts (aqueous, methanol, and hexane) of PS for 24 h. The concentrations were determined by MTT assay above. Then, cells were washed by ice-cold Dulbecco’s phosphate buffer saline (dPBS), scraped, harvested by centrifugation, and resuspended in 10 ml deionized water. Following ultrasonication at 4 °C for 15 min, an aliquot was taken out for protein determination by using Bradford (1976) method.
2.8. Measurement of MDA level
Lipid peroxidation was assayed by determining the production of thiobarbituric acid reactive substances (TBARS) and was expressed as MDA equivalents (Ohkawa et al., 1979). Its absorbance was determined at 532 nm spectrophotometrically (Shimadzu, Japan). The TBARS concentrations were extrapolated from the TEP serial dilution standard curve. The TBARS values were then stated as nmol MDA per µg protein in unit.
2.9. Measurement of SOD, CAT and GPX levels
SOD level was assayed as per previous protocol (Beyer and Fridovich, 1987). Briefly, each cell homogenate (20 µl) was added into the substrate mixture containing L-methionine, NBT, and Triton X-100 in dPBS. For control, it was replaced with 20 µl buffered solvent. Riboflavin (10 µl) was added to the mixture followed by illumination with two fluorescence lamp 20 W Sylvania Grolux for 7 min. The absorbance of each sample was measured at 560 nm spectrophotometrically (Shimadzu, Japan). The amount of SOD required to inhibit the rate of reduction by 50% was defined as one unit. CAT level was determined as previously described (Aebi, 1984). Briefly, the reactive mixture contained 1 ml H2O2 and 2 ml supernatant and the disappearance of H2O2 was monitored at 240 nm. GPX activity was assayed as per previous protocol (Lawrence and Burk, 1976). Briefly, each cell homogenate (20 µl) was added into the reactive mixture containing 1 mmol/L EDTA, 1 mmol/L NaN3, 0.2 mmol/L NADPH, 1 EU/ml glutathione reductase and 1 mmol/L GSH in 50 ml dPBS. After incubation at 37 °C for 5 min, H2O2 was added to initiate the reaction. The GPX level was measured as the rate of NADPH oxidation at 340 nm.
2.10. Statistical analysis
Statistical analysis was performed using a one-way analysis of variance (SPSS software version 12.0.1, Chicago, IL, USA). The results were expressed as mean±standard deviation (SD) of triplicate determination with P<0.05 considered as statistically significant.
3. Results
3.1. Antioxidant potential and total phenolics content of Piper sarmentosum
The FRAP and TPC results of PS crude extract are presented in Table 1. Each gram of PS extract has high FRAP value [(18.90±0.02) µmol Fe(II)/g DM] based on the FRAP assay. In addition, the PS extract also contained a high TPC [(90.86±0.37) mg GAE/g DM].
Table 1.
Antioxidant potential and total phenolics content of Piper sarmentosum
| FRAP value (µmol Fe(II)/g DM) | TPC value (mg GAE/g DM) | |
| PS | 18.90±0.02 | 90.86±0.37 |
| Vitamin C | 19.28±0.02 | 93.89±0.38 |
Values are mean±SD of three independent experiments. Vitamin C was used as a standard
3.2. Cell viability
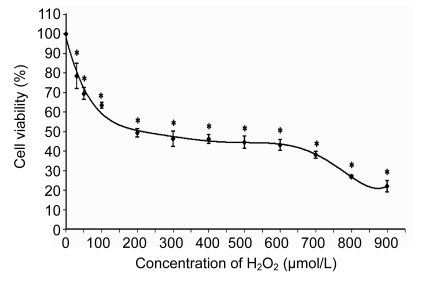
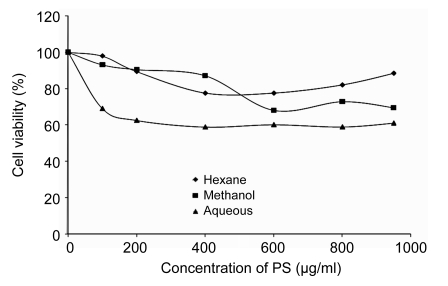
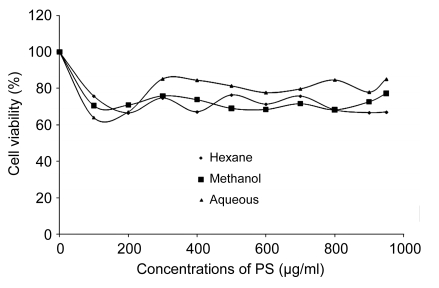
HUVECs were exposed to increasing concentrations of H2O2 from 0 to 1000 µmol/L for 24 h. Using MTT method, it was observed that the 50% inhibition (IC50) for H2O2 was (180±5) µmol/L which was used as positive control for the subsequent experiments. As shown in Fig. 1, the cytotoxicity effect of H2O2 on HUVECs was concentration-dependent. It was evident that H2O2 significantly decreased cell viability with increased concentrations. On the other hand, the cytotoxicity test of the three different extracts of PS with increasing concentrations used in this study was shown not to be toxic to HUVECs up to a concentration of 1000 µg/ml after 72-h incubation (Fig. 2). In addition, Fig. 3 also shows that the treatment of cells with three different extracts of PS in the presence of 180 µmol/L H2O2 protected the cells against the cytotoxicity effect of H2O2. The 50% effective concentration (EC50) of PS was 150 µg/ml as the cell survival rates were increased from 50% of H2O2-treated cells up to 70%. Therefore, four different concentrations (100, 150, 250, and 300 µg/ml) of the three different extracts of PS were used throughout this study.
Fig. 1.
Effect of H2O2 on cultured HUVECs after 24 h incubation
Values are mean±SD of three independent experiments. * P<0.05 compared with negative control group
Fig. 2.
Effects of aqueous, methanol, and hexane extracts of Piper sarmentosum on cultured HUVECs after 72-h incubation
Values are mean±SD of three independent experiments
Fig. 3.
Effects of aqueous, methanol, and hexane extracts of Piper sarmentosum in the presence of 180 µmol/L H2O2-induced HUVECs after 24-h incubation
Values are mean±SD of three independent experiment
3.3. MDA level
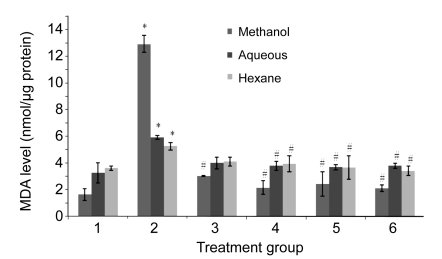
As shown in Fig. 4, the MDA level in the group treated with H2O2 was significantly increased compared to the untreated group. All the three groups treated with H2O2 and aqueous, methanol, and hexane extracts of PS simultaneously showed a significant reduction of the MDA level compared to the H2O2 group respectively except the 100 µg/ml aqueous and hexane extracts. No significant changes were observed between the extracts and the untreated group.
Fig. 4.
Effects of aqueous, methanol, and hexane extracts of Piper sarmentosum on the MDA level in H2O2-induced HUVECs
Treatment groups: 1, negative control; 2, 180 µmol/L H2O2 (positive control); 3, 100 µg/ml PS; 4, 150 µg/ml PS; 5, 250 µg/ml PS; 6, 300 µg/ml PS. Values are mean±SD of three independent experiments. * P<0.05 compared with negative control group; # P<0.05 compared with positive control group
3.4. SOD, CAT, and GPX levels
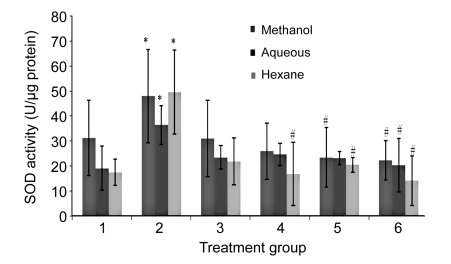
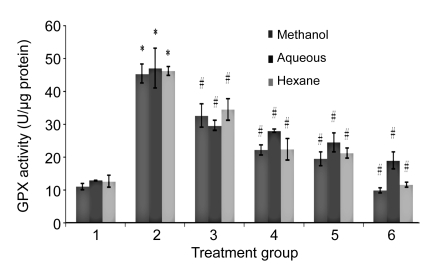
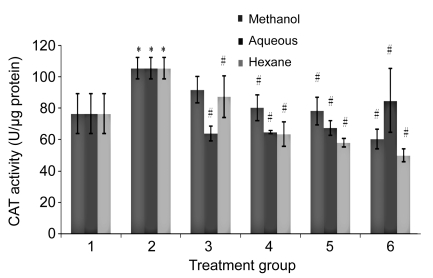
The SOD, CAT, and GPX levels in H2O2-treated HUVECs were significantly increased compared to the untreated group (Figs. 5–7). In contrast, the levels of enzymatic antioxidants of all PS treatment groups were significantly reduced compared to the H2O2 group, respectively. No significant changes were observed between all the concentrations of PS extracts and the untreated group.
Fig. 5.
Effects of aqueous, methanol, and hexane extracts of Piper sarmentosum on the SOD activity in H2O2-induced HUVECs
Treatment groups: 1, negative control; 2, 180 µmol/L H2O2 (positive control); 3, 100 µg/ml PS; 4, 150 µg/ml PS; 5, 250 µg/ml PS; 6, 300 µg/ml PS. Values are mean±SD of three independent experiments. * P<0.05 compared with negative control group; # P<0.05 compared with positive control group
Fig. 7.
Effects of aqueous, methanol, and hexane extracts of Piper sarmentosum on the GPX activity in H2O2-induced HUVECs
Treatment groups: 1, negative control; 2, 180 µmol/L H2O2 (positive control); 3, 100 µg/ml PS; 4, 150 µg/ml PS; 5, 250 µg/ml PS; 6, 300 µg/ml PS. Values are mean±SD of three independent experiments. * P<0.05 compared with negative control group; # P<0.05 compared with positive control group
Fig. 6.
Effects of aqueous, methanol, and hexane extracts of Piper sarmentosum on the CAT activity in H2O2-induced HUVECs
Treatment groups: 1, negative control; 2, 180 µmol/L H2O2 (positive control); 3, 100 µg/ml PS; 4, 150 µg/ml PS; 5, 250 µg/ml PS; 6, 300 µg/ml PS. Values are mean±SD of three independent experiments. * P<0.05 compared with negative control group; # P<0.05 compared with positive control group
4. Discussion
Oxygen-free radical-induced oxidative stress is implicated to cause membrane lipid peroxidation, membrane protein damage, altered antioxidant system, DNA mutation, altered gene expression, and apoptosis, thereby leading to cancer, cardiovascular disease, atherosclerosis and other neurodegenerative diseases (Estany et al., 2007; Renugadevi and Milton Praba, 2010). H2O2, one of the main ROS that freely diffuse inside and outside cells, is able to modulate multiple cellular processes, cell proliferation, signal transduction pathway, gene expression, DNA damage, apoptosis, and necrosis (Stone and Yang, 2006). In our experimental model, exposure of H2O2 to HUVECs had induced oxidative damage and loss of cell viability. This finding is in accordance with previous studies that reported the toxic effects of H2O2 on HUVECs and other cell types like human fibroblast cells, PC12 cells, endometrial cells, and bovine aorta endothelial cells (Spencer et al., 2001; Hou et al., 2004; Guan et al., 2006; Cianchetti et al., 2008; Estany et al., 2007; Chung et al., 2008).
Considerable interest has been focused on identifying antioxidant compounds that are pharmacologically potent with low or no side effect or toxicity. In the present study, the TPC presented in PS has strong ferric-reducing power that might influence the antioxidant potential of PS in ameliorating the harmful effects of H2O2. Furthermore, it is important to mention that HUVECs did not exhibit toxic effect even though the cells were exposed to high concentrations (1000 µg/ml) of aqueous, methanol, and hexane extracts of PS. This could highlight the safety use of PS on HUVECs.
The study by Subramaniam et al. (2003) had identified the natural antioxidant superoxide scavenger known as naringenin obtained from the methanol extract of PS, which showed a high antioxidative activity of 75.7% among other eight types of flavonoids studied. Another researcher had identified an aromatic alkaloid compound known as 1-nitrosoimino-2,4,5-trimethoxybenzene from the hexane extract of PS (Ee et al., 2009). Another previous study had identified myricetin, quercetin, and rutin in the aqueous-methanol extract of PS, which were more powerful antioxidants than vitamins C and E and β-carotene in in vitro lipoprotein oxidation model for heart disease (Vinson et al., 1995; Miean and Mohamed, 2001). It is also noted that those different active compounds can be extracted by various solvents. Thus, the aim of this study is to look into these three different solvents used for extraction, namely, aqueous, methanol, and hexane to ascertain the most appropriated extract that could exert the antioxidative effect.
In this experiment, crude extracts from PS with three different solvents (aqueous, methanol, and hexane) were used to treat H2O2-induced HUVECs. Therefore, a limitation of this study was the inability to determine the specific components of the plant that mediated the observed effects. We used three different solvents for extractions, and found that all of them exhibited similar antioxidant effects. However, the TPCs were found to be higher in the methanol and hexane extracts compared to the aqueous extract of PS, which is in accordance to previous research findings (Hussain et al., 2009a).
Lipid peroxidation is the degradative process that involves the chain reaction of free radicals with polyunsaturated fatty acids. This reaction leads to rearrangement of double bonds into degradative products such as MDA, and conjugated diene, as well as chemical modifications in the apolipoprotein B (Apo-B) protein (Upsani et al., 2001). In this study, the MDA level was increased in H2O2-induced HUVECs. The result was in line with previous studies that showed an increased MDA level following H2O2 addition in HUVECs (Wang Y.K. et al., 2005; Lin R. et al., 2006; Wang W.R. et al., 2006). Moreover, other ROS precursors like carbon tetrachloride (CCl4) and azoxymethane (AOM) were found to increase the MDA levels in the primary culture of rat hepatocytes and colon carcinogenesis in male mice, respectively (Lin Y.L. et al., 2006; Ashokkumar and Sudhandiran, 2008). These findings highlighted the importance of ROS as a mediator of cellular injury (Halliwell and Gutteridge, 1989; Valko et al., 2007). The MDA level in the methanol extract of PS was higher compared to the aqueous and hexane extracts, but the increase was insignificant likely due to partial overoxidation of the cells.
Treatment of H2O2-induced HUVECs concomitantly with aqueous, methanol, and hexane extracts of PS had prevented cell apoptosis and reduced formation of MDA, which showed the protective effect of PS on ROS and eventually on membrane damage. It was found that the aqueous extract of PS showed decreased cell viability as compared to the methanol and hexane extracts. It may be mentioned that the TPC is lower in the aqueous extract of PS compared to the methanol and hexane extracts. This might have been attributed to a decrease in cell viability compared to the methanol and hexane extracts. Although the cell viability for the aqueous extract of PS is lower, the decrease is insignificant.
The exact mechanism how PS acted as an antioxidant is still unclear. However, it is anticipated that PS would exhibit antioxidant effects against membrane lipid peroxidative damage by their ability to interact with and penetrate the lipid bilayers. The antiradical property of flavonoids in PS would also be ascribed to scavenge superoxide anion and hydroxyl radicals at the stage of initiation and termination. Our findings are in accordance with previous studies that reported flavonoid plants such as Epimedii Herba, Silybum marianum, and Salvia miltiorrhiza had reduced the MDA level in H2O2-induced HUVEC culture (Wang Y.K. et al., 2005; Lin R. et al., 2006; Wang W.R. et al., 2006). It also has been reported that there was a reduction of the MDA level after supplementation with luteolin in AOM-induced colon carcinogenesis of male mice (Ashokkumar and Sudhandiran, 2008).
The main constituents of PS are alkaloids, amides, pyrones, flavonoids, sterols, and neolignans (Hussain et al., 2009b). Flavonoids are a large group of naturally occurring plant-phenolic compounds that possess many biological and pharmacological actions against bacteria, allergy, virus, cancer, inflammation, and thrombosis (Seyoum et al., 2006). A positive correlation was found between antioxidant activity and total polyphenols, flavonoids and amides of PS in the β-carotene linoleate model and in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) model (Hussain et al., 2009a).
Flavonoids are believed to inhibit the activities of enzymes involved in the conversion of membrane polyunsaturated fatty acids (PUFAs) to active mediators such as phospholipase A2, cyclooxygenase, and lypoxygenase, and scavenge free radicals (Miean and Mohamed, 2001). Flavonoids effectively quench ROS due to their 4′-hydroxyl group in the β-ring that possesses electron-donating properties and is a radical target. Flavonoids are oxidized by radicals thereby forming more stable and less reactive radicals. Thus, flavonoids stabilize ROS by reacting with compounds that have radicals (van Acker et al., 2000).
SOD, CAT, and GPX are the most crucial enzymes in cellular antioxidant systems that play critical roles on the elimination of excess ROS in living organism. The SOD converts superoxide radical to hydrogen peroxide that is subsequently converted to water by CAT and GPX. Inadequate elimination of ROS results in oxidative stress that may cause severe metabolic malfunctions and damage to biological macromolecules (Mates, 2000). In the present study, H2O2-induced HUVECs increased the antioxidant enzymes levels. The results indicate that the production of higher levels of ROS generated by H2O2 treatment may have triggered the increased antioxidant enzymatic activities in the cells. It may be attributed to an instant active role of SOD, CAT, or GPX in modulating the harmful effects of H2O2.
Interestingly, another study had shown that the antioxidant enzyme levels were reduced in AOM-administered mouse colon tissues (Ashokkumar and Sudhandiran, 2008). This may be due to the aberrant increase in the levels of ROS, which enhanced the oxidative stress coupled with proliferation of colonocytes in colorectal malignant carcinoma. This result also correlates with the previous report that reported that the antioxidant enzyme levels were reduced due to overutilization to scavenge the products of lipid peroxidation as well as sequestration by tumor cell (Manju and Nalini, 2005).
Our results show that all concentrations of PS extracts reduced the levels of SOD, CAT, and GPX in H2O2-induced HUVECs. Previous studies had also indicated that the roots of Salvia miltiorrhiza had reduced the SOD level in HUVECs (Lin Y.L. et al., 2006). These findings could lead to a hypothesis that the flavonoids presented in PS initially played a role in reducing the ROS generated by H2O2 thus reducing the need for enhanced antioxidant enzymatic activities. Nevertheless, the extracts of Ligusticum chuanxiong and Angelica sinensis had suppressed production of ROS that may be associated with increased levels of SOD, CAT, and GPX in H2O2-induced HUVECs (ECV 304) (Hou et al., 2004). Luteolin supplementation also enhanced the levels of antioxidant enzymes by suppressing lipid peroxidation and oxidative stress (Ashokkumar and Sudhandiran, 2008).
To the best of our knowledge, this is the first study that discusses the use of PS extract in the prevention of atherosclerosis. The antioxidant properties of PS could be explained by its high flavonoid contents; however further experiments are needed to determine the main active components that are responsible for its antioxidant activity.
5. Conclusion
In conclusion, we demonstrated in the present study that the aqueous, methanol, and hexane extracts of PS exhibited potent effects as an antioxidant on modulating oxidative stress and preventing cell apoptosis in H2O2-induced HUVECs. Our data suggest that PS may be used as a safe supplement in reducing endothelial injury induced by oxidative stress.
Acknowledgments
The authors would like to express their sincere gratitude to Universiti Kebangsaan Malaysia Medical Centre and Hospital Kuala Lumpur for the supply of human umbilical cords and Forest Research Institute Malaysia for preparation of PS. The authors would like to thank Associate Professor Dr. Srijit DAS from Universiti Kebangsaan Malaysia for his invaluable comments in preparing the manuscript.
Footnotes
Project (Nos. UKM-FF-03-FRGS0005-2007 and FF-138-2007) supported by the Ministry of Higher Education and Universiti Kebangsaan Malaysia
References
- 1.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Ashokkumar P, Sudhandiran G. Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomed Pharmacother. 2008;62(9):590–597. doi: 10.1016/j.biopha.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 4.Beyer WFJr, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161(2):559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005;92(3):491–497. doi: 10.1016/j.foodchem.2004.07.035. [DOI] [Google Scholar]
- 7.Chung JE, Kim SY, Jo HH, Hwang SJ, Chae B, Kwon DJ, Lew YO, Lim YT, Kim JH, Kim EJ, et al. Antioxidant effects of equol on bovine aorta endothelial cells. Biochem Biophys Res Commun. 2008;375(3):420–424. doi: 10.1016/j.bbrc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Cianchetti S, Fiorentino AD, Colognato R, Di Stefano R, Franzoni F, Pedrinelli R. Anti-inflammatory and anti-oxidant properties of telmisartan in cultured human umbilical vein endothelial cells. Atherosclerosis. 2008;198(1):22–28. doi: 10.1016/j.atherosclerosis.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Ee GCL, Lim CM, Lim CK, Rahmani M, Shaari K, Bong CFG. Alkaloids from Piper sarmentosum and Piper nigrum . Nat Prod Res. 2009;23(15):1416–1423. doi: 10.1080/14786410902757998. [DOI] [PubMed] [Google Scholar]
- 11.Estany S, Palacio JR, Barnadas R, Sabes M, Iborra A, Martinez P. Antioxidant activity of N-acetylcysteine, flavonoids and α-tocopherol on endometrial cells in culture. J Reprod Immunol. 2007;75(1):1–10. doi: 10.1016/j.jri.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Guan S, Bao YM, Jiang B, An LJ. Protective effects of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur J Pharmacol. 2006;538(1-3):73–79. doi: 10.1016/j.ejphar.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Oxford University Press; 1989. [Google Scholar]
- 14.Hou YZ, Zhao GR, Yang J, Yuan YJ, Zhu GG, Hiltunen R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004;75(14):1775–1786. doi: 10.1016/j.lfs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Hussain K, Ismail Z, Sadikun A, Ibrahim P. Antioxidant, anti-TB activities, phenolic and amide contents of standardized extract of Piper sarmentosum Roxb. Nat Prod Res. 2009;23(3):238–249. doi: 10.1080/14786410801987597. [DOI] [PubMed] [Google Scholar]
- 16.Hussain K, Ismail Z, Sadikun A, Ibrahim P. Proximate analysis of different parts of Piper sarmentosum and quantification of total amides in various extracts. Phcog Res. 2009;2:113–119. [Google Scholar]
- 17.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human umbilical endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest. 1973;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71(4):952–958. doi: 10.1016/0006-291X(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 19.Lin R, Wang WR, Liu JT, Yang GD, Han CJ. Protective efficacy of tanshinone IIA on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanism. J Ethnopharmacol. 2006;108(2):217–222. doi: 10.1016/j.jep.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Lin YL, Lee TF, Huang YJ, Huang YT. Antiproliferative effect of salvianolic acid A on rat hepatic stellate cells. J Pharm Pharmacol. 2006;58(7):933–939. doi: 10.1211/jpp.58.7.0008. [DOI] [PubMed] [Google Scholar]
- 21.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogenic during the initiation, post-initiation stages of 1,2-dimethylhyrazine-induced colon cancer. Clin Chim Acta. 2005;358(1-2):60–70. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153(1-3):83–104. doi: 10.1016/S0300-483X(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 23.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49(6):3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Nagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Perry LM. Medicinal Plants of East and Southeast Asia. Cambridge: MIT Press; 1981. [Google Scholar]
- 26.Peungvicha P, Thirawarapa S, Temsiririrkkul R, Watanabe H, Prasain JK, Kadota S. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J Ethnopharmacol. 1998;60(1):27–32. doi: 10.1016/S0378-8741(97)00127-X. [DOI] [PubMed] [Google Scholar]
- 27.Pongboonrod S. The Medicinal Plants of Thailand. Bangkok, Thailand: Kasembanakit Press; 1976. [Google Scholar]
- 28.Pongmarutai M. Studying Antidiabetic Action of Piper rostratum. Bangkok, Thailand: Mahidol University; 1980. MS Thesis. [Google Scholar]
- 29.Renugadevi J, Milton Praba S. Cadmium-induced hepatoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol. 2010;62(2):171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 31.Seyoum A, Asres K, El-Fiky FK. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 2006;67(18):2058–2070. doi: 10.1016/j.phytochem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Spencer JP, Schroeter H, Kuhnle G, Srai SK, Tyrrell RM, Hahn U, Rice-Evans C. Epicatechin and its in vivo metabolite, 3′-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem J. 2001;354(3):493–500. doi: 10.1042/0264-6021:3540493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8(3-4):243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 34.Subramaniam V, Adenan MI, Ahmad AR, Shahdan R. Natural antioxidants: Piper sarmentosum (Kadok) and Morinda elliptica (Mengkudu) Mal J Nutr. 2003;9(1):41–51. [PubMed] [Google Scholar]
- 35.Takahashi S, Abe T, Gotoh J, Fukuuchi Y. Substrate-dependence of reduction of MTT: a tetrazolium dye differs in cultured astroglia and neurons. Neurochem Int. 2002;40(5):441–448. doi: 10.1016/S0197-0186(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 36.Upsani CD, Khera A, Balaraman R. Effect of lead and vitamin E, C or spiruline on malondialdehyde, conjugated dienes and hydroperoxides in rats. Indian J Exp Biol. 2001;39(1):70–74. [PubMed] [Google Scholar]
- 37.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Review: free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.van Acker FAA, Schouten O, Haenen GRMM, van der Vijgh WJF, Bast A. Flavonoids can replace α-tocopherol as an antioxidant. FEBS Lett. 2000;473(2):145–148. doi: 10.1016/S0014-5793(00)01517-9. [DOI] [PubMed] [Google Scholar]
- 39.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 1998;46(10):4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 40.Vinson JA, Dabbagh YA, Serry MM, Jang J. Plant flavonoids, especially tea flavonols are powerful antioxidants using an in vitro model for heart disease. J Agric Food Chem. 1995;43(11):2800–2804. doi: 10.1021/jf00059a005. [DOI] [Google Scholar]
- 41.Wang WR, Lin R, Peng N, Han CJ. The protective effects of tanshinone IIA on vascular endothelial cells injury induced by hydrogen peroxide. J Clin Med Mater. 2006;29:53–55. [Google Scholar]
- 42.Wang YK, Hong YJ, Huang ZQ. Protective effects of silybin on human umbilical vein endothelial cell injury induced by H2O2 in vitro. Vasc Pharmacol. 2005;43(4):198–206. doi: 10.1016/j.vph.2005.06.002. [DOI] [PubMed] [Google Scholar]