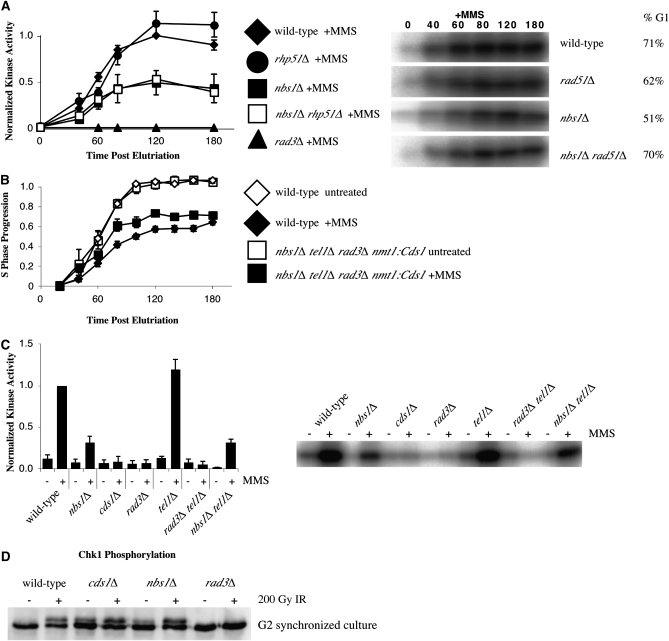
Figure 4.—
The nbs1Δ slowing defect is not a direct consequence of its checkpoint signaling defect. (A) Removing recombination does not rescue the checkpoint signaling defect exhibited by nbs1Δ cells. rhp51Δ (yFS556, n = 4, G1 enrichment range 51–83%) and wild-type (yFS162, data from 2A, n = 8, G1 enrichment range 54–92%) cells display similar checkpoint signaling in response to MMS while nbs1Δ (yFS267, data from 2A, n = 8, G1 enrichment range 28–91%) and the nbs1Δ rhp51Δ (yFS639, n = 5, G1 enrichment range 25–70%) double mutant display reduced checkpoint signaling. Representative raw data and G1 enrichment is shown. (B) Overproduction of Cds1 fails to rescue the slowing defect exhibited in the nbs1Δ tel1Δ rad3Δ nmt1:Cds1 mutant (yFS724). (C) Tel1 is not required for Cds1 activation in response to MMS during S phase as measured by asynchronous kinase assay. Wild type (yFS105) displays strong Cds1 activity while cds1Δ (yFS199), rad3Δ (yFS270), and rad3Δ tel1Δ (yFS723) mutants all display a complete defect in Cds1 activation. The nbs1Δ (yFS249) and nbs1Δ tel1Δ (yFS730) mutants display a partial signaling defect and tel1Δ displays strong Cds1 activation. (D) Nbs1 is not involved in phosphorylation of the G2 DNA damage checkpoint kinase Chk1. The nbs1Δ strain (yFS639) displays wild-type levels of phosphorylation of Chk1 in response to 200 Gy ionizing radiation when compared with the rad3Δ (yFS690), cds1Δ (yFS299), and wild-type (yFS06) controls.