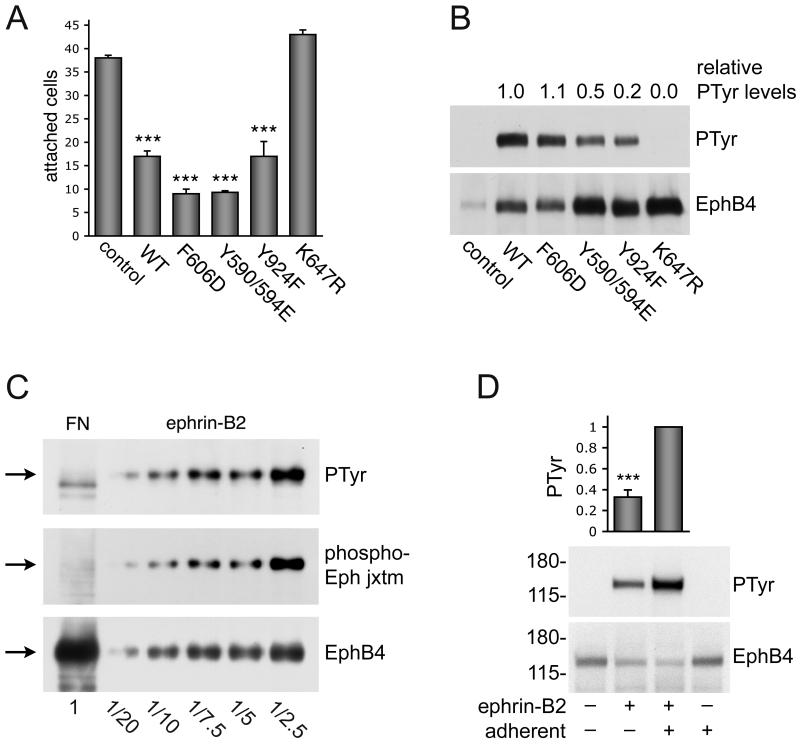
Figure 6. Inhibition of cell adhesion by EphB4 requires kinase activity.
(A) MDA-MB-435p cells, which express low levels of EphB4, were transfected with the indicated EphB4 constructs or pcDNA3 control vector at a 10:1 ratio with EGFP. Forty-eight hours after transfection, the cells were detached and allowed to adhere to fibronectin for 15 min. EGFP-positive cells that had attached to fibronectin were counted under a fluorescence microscope. The histogram shows averages ± SEM from triplicate measurements.***P<0.001 compared to vector control using one-way ANOVA and Tukey’s post-hoc test. Furthermore, the EphB4 F606D and Y590/594E mutants inhibited cell attachment slightly more than EphB4 wild-type (*P<0.05). (B) 293 HEK cells were transfected with the indicated EphB4 constructs or vector control. EphB4 immunoprecipitates were probed with anti-phosphotyrosine antibodies (PTyr) and reprobed with anti-EphB4 antibodies. The tyrosine phosphorylation levels were quantified from immunoblots and normalized to the amount of immunoprecipitated EphB4. The numbers above the blot represent phosphorylation levels compared to wild-type EphB4 averaged from 2 independent experiments. (C) EphB4 immunoprecipitates from MDA-MB-435c cells plated on fibronectin (FN) or treated with 3 μg/ml clustered ephrin-B2 Fc for 15 min were probed with antibodies to the two conserved Eph receptor juxtamembrane tyrosine phosphorylation sites (phospho-Eph jxtm), reprobed with anti-phosphotyrosine antibodies (PTyr) and then reprobed with anti-EphB4 antibodies. Different fractional amounts of the immunoprecipitate from ephrin-B2-treated cells were loaded, as indicated at the bottom, to show that even the low levels of phosphorylated EphB4 present in a small portion (1/20) of the immunoprecipitate are detectable. (D) Integrin-mediated adhesion potentiates ephrin-induced tyrosine phosphorylation of EphB4. Serum-starved MDA-MB-435c cells were kept adherent or detached and kept in suspension for 30 min. Cells were then stimulated for 20 min with 1.5 μg/ml clustered ephrin-B2 Fc. EphB4 immunoprecipitates were probed with anti-phosphotyrosine antibodies and reprobed with anti-EphB4 antibodies. The histogram shows average relative levels of EphB4 phosphorylation normalized to the amount of immunoprecipitated EphB4 from 6 independent experiments. Error bars represent SEMs. ***P<0.001 by Student’s t-test.