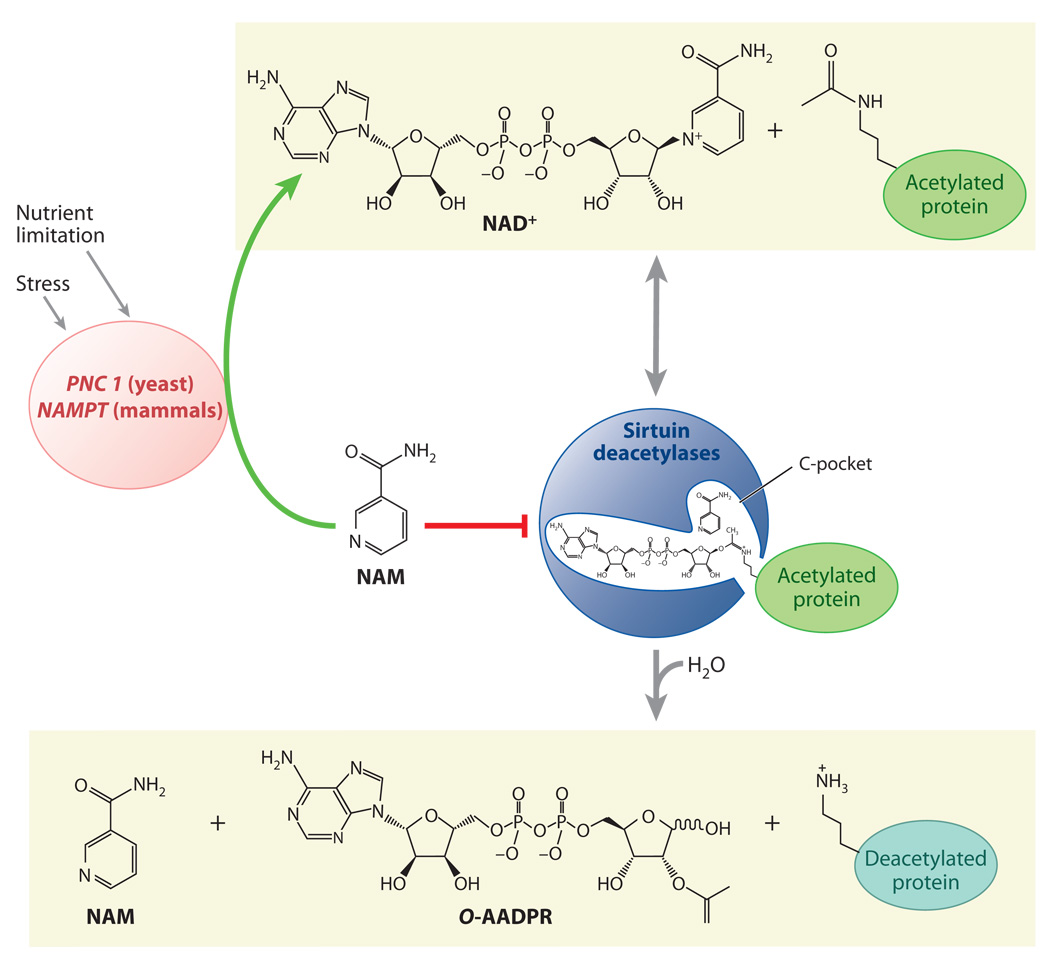
Figure 1.
The sirtuin deacetylation reaction and regulation by stress and nutrition. Unlike type I and II deacetylases, which hydrolyze the acetyl group on a substrate, sirtuin deacetylases catalyze an unprecedented two-step biological reaction that consumes nicotinamide adenine dinucleotide (NAD+) and releases nicotinamide (NAM), O-acetyl-ADP-ribose (AADPR), and the deacetylated substrate. Amide-to-ester acyltransfer is unfavorable, but hydrolysis of NAD+ can provide a favorable driving force for the overall sirtuin reaction. Evidence favors a mechanism in which electrophilic capture of the acetyl oxygen in an ADP-ribosyltransfer reaction forms an ADP-ribose peptide-imidate complex. This intermediate may last for a few seconds, enough time for NAM to enter the C-pocket and catalyze a reverse reaction. Activation of sirtuins can be facilitated by the removal of NAM and its conversion to NAD+ by PNC1 (yeast and simple metazoans) or NAMPT (mammals and alpha proteobacteria), two genes upregulated by stress and nutrient limitation.