Abstract
A promising, environmentally safe method for inactivating fungal spores of Penicillium digitatum, a difficult-to-inactivate food spoilage microorganism, was developed using a high-density nonequilibrium atmospheric pressure plasma (NEAPP). The NEAPP employing Ar gas had a high electron density on the order of 1015 cm−3. The spores were successfully and rapidly inactivated using the NEAPP, with a decimal reduction time in spores (D value) of 1.7 min. The contributions of ozone and UV radiation on the inactivation of the spores were evaluated and concluded to be not dominant, which was fundamentally different from the conventional sterilizations.
Inactivation of microorganisms using a plasma processing method has attracted much attention recently, especially as a substitute for medical instrument sterilization methods. A plasma processing method possesses many advantages such as a low-temperature treatment and short processing time. Plasma inactivation shows promise as a very effective system, which causes minimal damage to the instruments.
Microorganisms were sterilized successfully using plasma processing. Inactivation factors, such as ultraviolet-C (UV-C) emission, charged species, neutral species, and synergic effects, for various plasma processes from low pressure to atmospheric pressure have been intensively studied.1, 2, 3, 4, 5, 6 However, there is no report based on the quantitative diagnostics on the gas phase and the inactivation.
In agricultural fields and plant protection stations, pesticides are sprayed to protect crops from various insects and viruses. Fungi, such as Aspergillus or Penicillium, contaminate foods, such as cereals, fruits, vegetables, and meat.7 The green mold of citrus is caused by spores of Penicillium digitatum, which is a difficult-to-inactivate postharvest disease. Methyl bromide is an effective and widely used pesticide. In 2005, however, it was prohibited because of its high ozone depletion potential. Residual agricultural chemicals8 also are harmful to the human body and the environment. The inactivation should cause minimal damage to crops, humans, and the environment, and should be easy to use. Spores of P. digitatum are different from other microorganisms for medical applications because of the resistant structure, composition, and function of their cell wall. For example, the larger dose for 90% inactivation of spores such as Penicillium by UV-C irradiation is required in comparison with the bacteria such as Bacillus and Escherichlia coli.9 However, the effect of NEAPP treatment on fungal spores has not been reported yet and the quantitative evaluation on the resistances against inactivation factors such as radicals are never known.
Nonequilibrium atmospheric pressure plasmas (NEAPPs) have many advantages; including the capability of being used outside a vacuum and not generating heat-induced damage. Because the energy of charged species is very low and densities are negligibly smaller than those of neutral radicals in the remote plasma region. In this study, the successful inactivation of the spores of P. digitatum using high-density NEAPP is reported. We also investigated the resistance against the UV radiation and ozone. The ozone density was measured by ultraviolet absorption spectroscopy (UVAS) and the effect of ozone was compared the ozonizer with the NEAPP quantitatively.
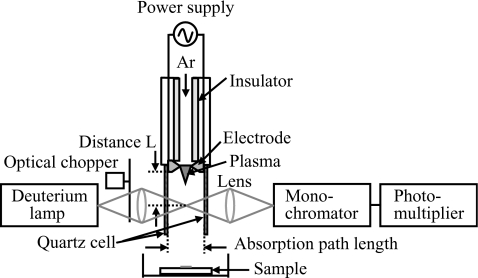
A schematic diagram of plasma inactivation is shown in Fig. 1. In this system, alternative current of 6 kV, transformed from 100 V and 60 Hz commercial power line, was applied to two electrodes, and Ar gas of 3 slm was flowed. The electron density of the NEAPP was approximately 1015 cm−3,10 it was two orders of magnitude greater than those of the conventional atmospheric pressure plasmas, which led to the generation of high-density reactive species.
Figure 1.
Schematic diagram of nonequilibrium atmospheric pressure plasma for plasma inactivation of Penicillium digitatum with an optical system for ultraviolet absorption spectroscopy.
Spores of P. digitatum were diluted by sterilized water including the surface-active agent for avoiding the clump of spores. Diluted spores were placed on a cover glass. The sample was set at distances (from the electrodes to the samples) of greater than 10 mm with the sample surface temperatures less than 60 °C in order to avoid heat damage of the samples from the plasma and evaluate the resistances against ozone and UV radiation precisely. In this case, the gas temperature of the plasma was about 1100 K near the electrodes. However, the length of the plasma torch was less than 4 mm from the electrodes and the plasma did not reach to the samples, so that the heat damage to the P. digitatum was eliminated. After NEAPP exposure, the samples were cultured on potato dextrose agar medium in an incubator for 72 h at 25 °C.
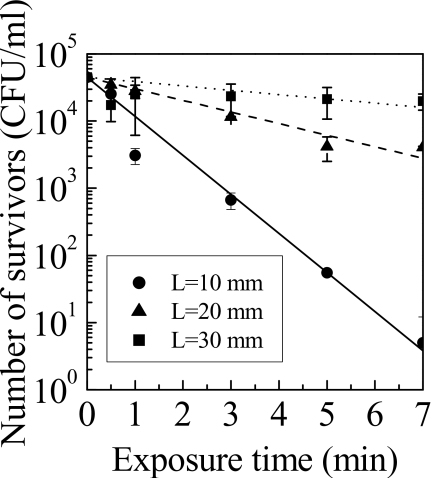
The number of spores that survived after exposure is shown in Fig. 2. The decimal reduction time of spores (D value) of this system at distances L of 10 mm, 20 mm, and 30 mm were 1.7 min, 5.8 min, and 15.6 min, respectively. The D value of 1.7 min is as fast as other microorganism sterilization procedures, indicating that this NEAPP is a high potential for inactivation of P. digitatum.
Figure 2.
Number of survivors as a function of distance from the electrode.
In general, ozone generators and UV lamps are used for inactivation. Therefore, it is important to investigate the inactivation effects of ozone and UV radiation. Absorption spectroscopy is a powerful tool to monitor the absolute density of species and has clarified plasma chemistry and the mechanism of etching or deposition.11, 12, 13 To clarify the inactivation effect of ozone, the absolute density of ozone was measured by UVAS and inactivation rate was compared to that from an ozonizer. In addition, the contribution of UV radiation to inactivation was investigated.
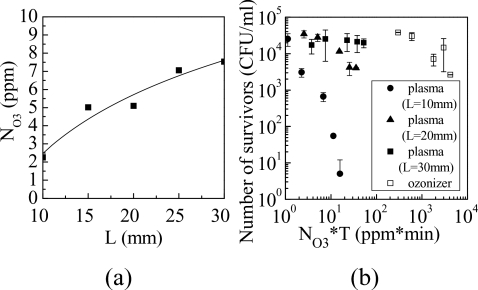
The absolute density of ozone in the NEAPP was measured by UVAS as shown in Fig. 1. A quartz cell was placed around the plasma to ensure that the absorption length was constant at 10 mm. Ozone has its maximum absorption cross-section of 1.15×10−17 cm2 at a wavelength of 254 nm.14 The absolute densities of ozone as a function of L, which was varied from 10 to 30 mm, are shown in Fig. 3a. The ozone density (NO3) increased from 2 to 8 ppm with an increase in L, while the inactivation rate decreased as shown in Fig. 2. The main production mechanism of ozone consists of two processes: the dissociation of oxygen molecules by electron impact, and the recombination of oxygen molecules and oxygen radicals via three-body collisions.1, 15 In contrast, the main loss mechanism is reaction with oxygen atoms.
Figure 3.
(a) Absolute density of ozone as a function of L; (b) Number of survivors as a function of integrated number density of ozone.
To evaluate the effect of ozone, the inactivation rate of the NEAPP was compared to that of an ozonizer, which produced ozone with a density of 600 ppm at a flow rate of 5 slm. The number of P. digitatum spore survivors after inactivation as a function of the integrated number density of ozone (NO3*T), the product of ozone density (NO3) multiplied by exposure time (T), is shown in Fig. 3b. In contrast, the D value of the system at L of 10 mm was 1.7 min, while that of the ozonizer was 6.1 min. Thus, the D value of this system was three times smaller than that of the ozonizer. Moreover, the NO3*T of the ozonizer was approximately two orders of magnitude larger than that of this system. Therefore, the contribution of ozone to inactivation of P. digitatum was small.
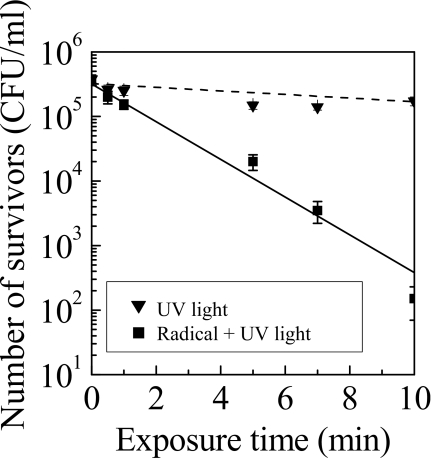
The UV-C emission originating from NO or N2 was observed in this system. To investigate the inactivation effect of UV radiation on P. digitatum, quartz was placed over the Petri dish. The quartz allows most UV light to pass through. This allows the contribution of UV emission on inactivation to be evaluated because the quartz allows only UV light to reach the samples, and prevents species such as ozone and radicals from reaching the samples. The numbers of survivors with and without quartz, corresponding to inactivation by only UV light exposure and by the simultaneous exposure of UV light and reactive species, are shown in Fig. 4. The sample was placed at L of 20 mm. The D values with and without quartz were 26.2 min and 3.8 min, respectively. The inactivation rate produced by exposure to UV and reactive species was approximately sevenfold greater than that produced by UV radiation alone. These results indicate that UV radiation does not contribute significantly to inactivation because of absorption by ambient gas atoms and molecules but reactive species play a major role in inactivation, which is fundamentally different from the conventional sterilizations using low pressure plasmas.1, 3, 4
Figure 4.
Number of survivors inactivated by UV radiation alone and by exposure to UV radiation and reactive species.
In summary, spores of P. digitatum were successfully inactivated using high-density NEAPP. Results from studies on the inactivation effects of ozone and UV radiation showed that the inactivation rate of this system was nearly three orders higher than that of an ozonizer using the integrated number density of ozone. Moreover, the contribution of UV radiation toward inactivation was not dominant for P. digitatum inactivation by NEAPP. There are other effects such as electric field and charged particle but it is assumed these effects are small because the inactivation was performed under the remote plasma region. The contribution of the reactive species such as OH radical, O radical and their by-product would be larger. Additional studies are necessary for evaluating the contributions of individual reactive species and performing the food and agricultural applications with high throughput and lower temperatures.
Acknowledgments
This work was partly supported by the Knowledge Cluster Initiative (Second Stage)-Tokai Region Nanotechnology Manufacturing Cluster and a Grant-in-Aid for Scientific Research on Innovative Areas “Frontier science of interactions between plasmas and nanointerfaces” (Grant No. 21110006) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- Fridman A., Plasma Chemistry (Cambridge University Press, New York, 2008). 10.1017/CBO9780511546075 [DOI] [Google Scholar]
- Deng X., Shi J., and Kong M. G., IEEE Trans. Plasma Sci. 34, 1310 (2006). 10.1109/TPS.2006.877739 [DOI] [Google Scholar]
- Moisan M., Barbeau J., Moreau S., Pelletier J., Tabrizian M., and Yahia L’H., Int. J. Pharm. 226, 1 (2001). 10.1016/S0378-5173(01)00752-9 [DOI] [PubMed] [Google Scholar]
- Laroussi M., Plasma Processes Polym. 2, 391 (2005). 10.1002/ppap.200400078 [DOI] [Google Scholar]
- Wintenberg K. K., Hodge A., Montie T. C., Deleanu L., Sherman D., Roth J. R., Tsai P., and Wadsworth L., J. Vac. Sci. Technol. A 17, 1539 (1999). 10.1116/1.581849 [DOI] [Google Scholar]
- Opretzka J., Benedikt J., Awakowicz P., Wunderlich J., and von Keudell A., J. Phys. D 40, 2826 (2007). 10.1088/0022-3727/40/9/024 [DOI] [Google Scholar]
- Rundberget T., Skaar I., and Flaaoeyen A., Int. J. Food Microbiol. 90, 181 (2004). 10.1016/S0168-1605(03)00291-5 [DOI] [PubMed] [Google Scholar]
- Kinay P., Mansour M. F., Gabler F. M., Margosan D. A., and Smilanick J. L., Crop Prot. 26, 647 (2007). 10.1016/j.cropro.2006.06.002 [DOI] [Google Scholar]
- Illuminating Engineering Society, IES Lighting Handbook: The Standard Lighting Guide, 2nd ed. (Illuminating Engineering Society, New York, 1952). [Google Scholar]
- Iwasaki M., Inui H., Matsudaira Y., Kano H., Yoshida N., Ito M., and Hori M., Appl. Phys. Lett. 92, 081503 (2008). 10.1063/1.2885084 [DOI] [Google Scholar]
- Takashima S., Hori M., Goto T., Kono A., Ito M., and Yoneda K., Appl. Phys. Lett. 75, 3929 (1999). 10.1063/1.125497 [DOI] [Google Scholar]
- Nagai H., Takashima S., Hiramatsu M., Hori M., and Goto T., J. Appl. Phys. 91, 2615 (2002). 10.1063/1.1435825 [DOI] [Google Scholar]
- Ohta T., Ito M., Tachibana Y., Taneda S., Takashima S., Hori M., Kano H., and Den S., Appl. Phys. Lett. 90, 251502 (2007). 10.1063/1.2751104 [DOI] [Google Scholar]
- Katrib Y., Martin S. T., Hung H. M., Rudich Y., Zhang H., Slowik J. G., Davidovits P., Jayne J. T., and Worsnop D. R., J. Phys. Chem. A 108, 6686 (2004). 10.1021/jp049759d [DOI] [Google Scholar]
- Eliasson B., Hirth M., and Kogelschatz U., J. Phys. D 20, 1421 (1987). 10.1088/0022-3727/20/11/010 [DOI] [Google Scholar]