Figure 3.
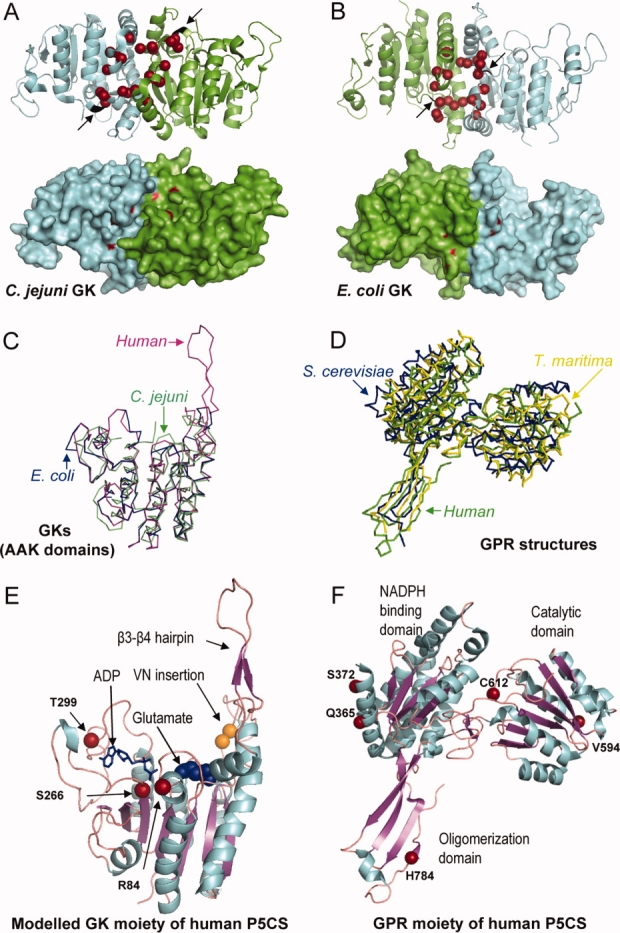
(A) A ribbon and surface representation of a GK dimer from C. jejuni and (B) from E. coli where the mutated residues found in the different GK and P5CS enzymes affecting proline regulation are drawn in red. Arrows denote the location of the VN insertion (colored in black) found in the human long P5CS isoform. C: Superposition of the AAK domain of E. coli GK colored in blue (2J5T), C. jejuni GK (2AKO) colored in green, together with the modeled human GK moiety generated by using the Swiss Model Workspace, colored in pink. D: Superposition of the GPR enzymes from T. maritima (1O20) colored in yellow, S. cerevisiae (1VLU) colored in blue and the human GPR moiety of P5CS (2H5G) colored in green. E: Ribbon representation of the modeled structure of the human GK moiety, with the ADP molecule depicted by sticks, and the glutamate by spheres, and (F) the crystal structure of the human GPR moiety with the different domains, the catalytic cysteine and some structural features indicated. The residues affecting clinical mutations and polymorphisms are shown.
