Table III.
Parameters determined from the 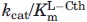 versus pH profiles of wild-type eCBL and the R58A, R58K and R372K site-directed mutantsa
versus pH profiles of wild-type eCBL and the R58A, R58K and R372K site-directed mutantsa
| Enzyme | pKa1 | pKa2 |
|---|---|---|
| eCBL | 8.28 ± 0.06 | 10.20 ± 0.06 |
| R58A | 7.93 ± 0.08 | 9.53 ± 0.09 |
| R58K | 8.94 ± 0.09 | 9.8 ± 0.1 |
| R372K | 8.44 ± 0.09 | 9.8 ± 0.1 |
Kinetic measurements for the eCBL-catalyzed hydrolysis of l-Cth were carried out from pH 6.4–10.6 in MBP buffer containing 0.01–6.25 mM l-Cth, 20 μM PLP, 2 mM DTNB and 0.068–4.5 μM enzyme at 25°C. The kcat/ versus pH data were fitted to equation 2 to obtain the values for pKa1 and pKa2.
versus pH data were fitted to equation 2 to obtain the values for pKa1 and pKa2.
