Figure 8.
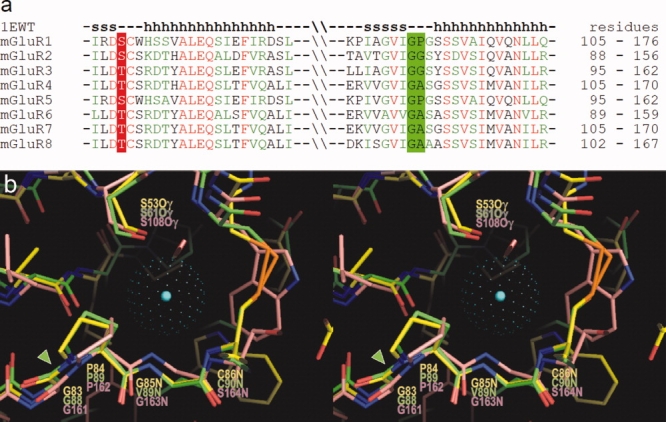
(a) Amino acid sequence alignment among mGluRs in the region homologous to the chloride binding site in ANPR. Residues in mGluRs, corresponding to Ser53 in ANPR, are conserved as either Ser or Thr. The Gly-Pro sequence in ANPR is conserved as Gly-Pro, Gly-Gly, or Gly-Ala. The secondary structures in mGluR1 are shown above the aligned sequences. (b) Overlay of the three-dimensional structures of the chloride binding sites in ANPR (PDB 1DP4)15 and NPCR (1JDP)16 and that of the water bound site in mGluR1 (1EWT)32 in stereo view. The carbon atoms in ANPR, NPCR, and mGluR1 are shown in yellow, green, and pink, respectively. The center of chloride atom in the ANPR is shown by a blue dot, and the van der Waals radius is presented by green dots. The conserved Gly-Pro cis-peptide bonds are highlighted by a green arrowhead. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
