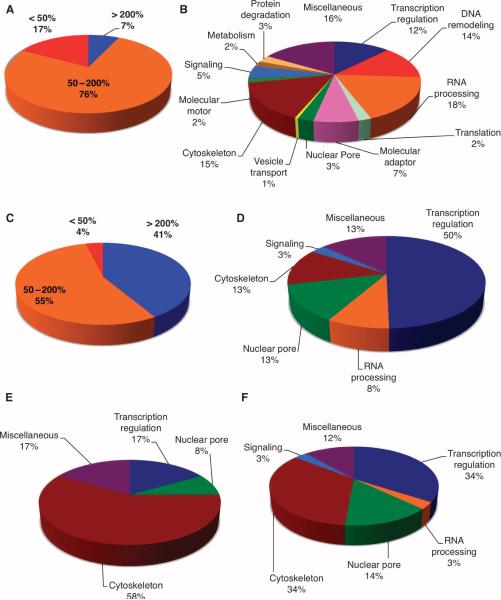
Fig. 3.
OGT overexpression triggers changes in mitotic phosphorylation and O-GlcNAcylation. (A) Phosphopeptides were enriched from pooled spindle-midbody extracts by purification over a titanium dioxide column. OGT overexpression decreased phosphorylation by more than 50% at 17% of the identified phosphopeptides, whereas only 7% of phosphorylation sites showed an increase of more than 200%. (B) Identified phosphorylation sites (397) occur on proteins that fall into similar functional classes. (C) Of the identified O-GlcNAcylation sites, 41% showed increased site modification of more than 200% in preparations from cells overexpressing OGT compared to cells overexpressing GFP, whereas only 4% of the O-GlcNAc sites showed decreased modification by more than 50%. (D) The GlcNAc-modified sites were predominately found on transcriptional regulators; however, RNA processing, nuclear pore, and cytoskeleton proteins also contained a large number of GlcNAcylation sites. (E) Functional classes of proteins whose O-GlcNAc site is also a reciprocal phosphorylation site. (F) Functional class of proteins that have a phosphorylation site within ± 10 amino acids proximal to an O-GlcNAcylation site.