Abstract
The ability to make advantageous choices among outcomes that differ in magnitude, probability, and delay until their arrival is critical for optimal survival and well-being across the lifespan. Aged individuals are often characterized as less impulsive in their choices than their young adult counterparts, demonstrating an increased ability to forgo immediate in favor of delayed (and often more beneficial) rewards. Such “wisdom” is usually characterized as a consequence of learning and life experience. However, aging is also associated with prefrontal cortical dysfunction and concomitant impairments in advantageous choice behavior. Animal models afford the opportunity to isolate the effects of biological aging on decision making from experiential factors. To model one critical component of decision making, young adult and aged Fischer 344 rats were trained on a two-choice delay discounting task in which one choice provided immediate delivery of a small reward and the other provided a large reward delivered after a variable delay period. Whereas young adult rats showed a characteristic pattern of choice behavior (choosing the large reward at short delays and shifting preference to the small reward as delays increased), aged rats maintained a preference for the large reward at all delays (i.e. – attenuated “discounting” of delayed rewards). This increased preference for the large reward in aged rats was not due to perceptual, motor, or motivational factors. The data strongly suggest that, independent of life experience, there are underlying neurobiological factors that contribute to age-related changes in decision making, and particularly the ability to delay gratification.
Keywords: aging, decision-making, choice, impulsivity, delay discounting, rat
1. Introduction
People are faced with numerous decisions throughout their lives, the consequences (outcomes) of which significantly impact their survival and quality of life. Often such decisions include temporal considerations, in which a delay imposed between a choice and the arrival of its corresponding outcome can substantially alter the desirability of that outcome. For example, individuals will virtually always choose a large over a small reward when the delay to delivery of the large reward is short; however, as the delay to the large reward increases, its subjective value decreases, or is “discounted”, making the small reward more preferable. This phenomenon, known as delay discounting, is well documented in a variety of settings, and all subjects reliably demonstrate it to some degree [1,22,39,49]. However, the degree of discounting can be modified substantially by factors including pharmacological agents, genetic background, and age [2,9-11,21,26,37,43].
Children and adolescents discount delayed rewards to a greater degree than adults (i.e. - the “impulsivity of youth” - [21,22]). However, surprisingly little is known about how more advanced ages affect this component of decision making, an issue of increasing importance as average life expectancy continues to rise [17]. Anecdote suggests that aged individuals are less impulsive than their younger counterparts. Consistent with this perception, Green and colleagues [21,22] reported that 70 year-olds discount delayed rewards to a lesser extent than do 20 year-olds. In contrast, behavioral economic models suggest that aged individuals should consider their reduced number of remaining years when making decisions, and therefore should discount delayed rewards to a greater extent than younger individuals [44]. In agreement with such models, some aged subjects show disadvantageous choice behavior in laboratory tests of decision making such as the Iowa Gambling Task [14,18]. Furthermore, functional studies in both animal and human subjects show that prefrontal cortical areas (particularly orbitofrontal cortex) involved in decision making processes may be compromised at advanced ages [28,29,42,47].
The influence of environmental factors on delay discounting [15,22,36] renders comparisons between young adult and aged humans difficult to interpret. For example, among many possible interpretations, such age-related differences in delay discounting could reflect differing degrees of opportunity for learning about the benefits of waiting for rewards (i.e. – learning that “patience has its virtues”), and/or even differences in socioeconomic status [22]. The current study was designed to minimize the influence of prior experience on decision making in aging using a rat model of human cognitive aging in which experiential factors (prior learning, environmental history) are controlled in young adult and aged subjects. Rats were trained in a two-choice delay discounting task, which pitted choice of a small immediate reward against a large reward delivered after a delay period. Aged subjects demonstrated a strong preference for the larger reward, irrespective of delay, providing support for a neurobiological (rather than solely experiential) explanation of decision making differences across the lifespan.
2. Materials and Methods
2.1. Subjects
Male Fischer 344 rats were used for this study. Aged (n=8) and young adult (n=8) rats were a mean of 24.72 (SEM = .16) and 6.11 (SEM = .03) months old, respectively, at the onset of behavioral testing. Young adult rats of this age were chosen because it is well past the age of sexual maturity (approximately 2-3 months). Fischer 344 rats are considered “aged” at 24 months, (with an average lifespan of 26.6 months and a maximum lifespan of approximately 30 months). By 24 months, a considerable proportion of the F344 population displays cognitive deficits on a range of behavioral tasks [3,20,30,55]. Rats were obtained from the National Institute on Aging colony and housed in the vivarium in the Psychology Building at Texas A&M University. This AALAC-accredited facility was maintained at a consistent 25°C with a 12:12 hour light/dark cycle (lights on at 0800), and rats had free access to food and water except as noted below. All rats in the study were screened daily for health problems including, but not limited to, cataracts, jaundice, food and water intake, and the appearance of tumors. Sentinel rats, housed alongside the rats used in this study, were routinely health screened and found to be negative for a range of pathogens. Upon autopsy, each subject was screened for visible pituitary tumors that could impair visual acuity by impinging on the optic nerve. No subjects were excluded from the study for health reasons, although two aged rats ceased responding during the control experiments that were conducted after the completion of the delay discounting procedure in Experiment 1. These rats were removed from the study at those points and excluded from subsequent analyses (one was removed during Experiment 2 and one was removed during Experiment 3). All animal procedures were conducted in accordance with approved institutional animal care procedures and NIH guidelines.
2.2 Apparatus
Testing was conducted in 6 identical standard rat test chambers (30.5 × 25.4 × 30.5 cm; Coulbourn Instruments, Allentown, PA) with metal front and back walls, transparent Plexiglas side walls, and a floor composed of steel rods (0.4 cm in diameter) spaced 1.1 cm apart. Each test chamber was housed in a sound attenuating cubicle, and equipped with a recessed food pellet delivery trough fitted with a photobeam to detect head entries and a 1.12 W lamp to illuminate the food trough. This trough, into which the 45 mg grain-based food pellet rewards (PJAI, Test Diet: Richmond, IN) were delivered, was located 2 cm above the floor in the center of the front wall. Two retractable levers were located to the left and right of the food delivery trough, 11 cm above the floor. Experiments were controlled and data collected by a computer interfaced with the behavioral test chambers and equipped with Graphic State 3.01 software (Coulbourn Instruments).
2.3. Experimental Procedures
The procedures for the delay discounting task have been described previously [16,43]. Prior to the start of behavioral testing, rats were reduced to 85% of their free-feeding weight over the course of one week, and were maintained as such throughout the duration of the experiment. On the day before the start of behavioral testing, each rat was given five 45 mg food pellets in its home cage to reduce neophobia to the food reward used in the task.
2.3.1. Shaping
The task began with a 64 minute session of magazine training consisting of 38 deliveries of a single food pellet with an inter-trial interval (ITI) of 100 ± 40 s. On the following day, the rats were shaped to press a single lever (either left or right, counterbalanced across groups; the other was retracted during this phase of training) in order to receive a single food pellet. Once they reached a criterion of 50 lever presses during a 30 minute session, they were shaped to press the opposite lever using the same schedule and criterion.
Following completion of lever press shaping, both levers were retracted, and rats were shaped to nose poke into the food trough during simultaneous illumination of the trough light and a 1.12 W house light. When a nose poke occurred, a single lever was extended, and a lever press resulted in immediate delivery of a single food pellet. Immediately following the lever press, the house and trough lights were extinguished and the lever was retracted. The left and right levers were presented an equal number of times, with no more than two consecutive presentations of the same lever. Rats were trained to a criterion of at least 60 successful trials in an hour with an ITI of 40 ± 10 s.
2.3.2. Experiment 1: Assessing Age-related Differences in Delay Discounting
Each session in the delay discounting task consisted of 5 blocks of 12 trials each. Within each session, each of the 100 s trials began with a 10 s illumination of the food trough and house lights. A nose poke into the food trough during this time extinguished the food trough light and triggered extension of either a single lever (forced trials) or of both levers simultaneously (choice trials). Trials on which rats failed to nosepoke during this time window were scored as omissions. A press on one lever (either left or right, counterbalanced across groups) resulted in delivery of a single food pellet immediately following the lever press. A press on the other lever resulted in delivery of four food pellets after varying delays. Once either lever was pressed, both levers were retracted and the house light was extinguished until food delivery. Food delivery was accompanied by the re-illumination of both lights, which were again extinguished upon entry to the food trough to collect the food or after 10 s, whichever occurred sooner. Each of the five 12 trial blocks in a session began with two forced trials (one for each lever), followed by 10 choice trials. During the first 12-trial block, the delay to the large reward was set at 0 s. In subsequent 12-trial blocks, the delay to the large reward increased to 10, 20, 40, and 60 s. Note that because trial durations were of fixed duration, reward choice did not influence the rapidity of progress through the trials (e.g. – choice of the small immediate reward did not result in sooner choice opportunities). Thus, choice of the large delayed reward was, from an objective perspective, an “optimal” choice, as it resulted in more food delivery. The percentage of trials on which rats chose the large reward lever (number of large reward lever choices/total responses) was calculated for each block as an indicator of reward preference [10,43]. Both young and aged rats were tested in the delay discounting task for 40 sessions, at which point stable responding was observed across the final 5 sessions (36-40). Stable responding was defined by the absence of a main effect of days in a repeated measures ANOVA, accompanied by a significant main effect of delay [10,43].
2.3.3. Experiment 2: Reversed order of presentation of delays
To determine whether the order of presentation of the delays across trial blocks affected choice behavior or the number of completed trials, the order of the delay blocks was reversed. Thus, the delays began at 60 s in the first block, and decreased to 40, 20, 10, and 0 s in each subsequent block of trials in a session. All other experimental conditions remained consistent with the procedures in Experiment 1. Rats were run in this condition for 13 sessions, at which point stable responding was observed across the final 5 sessions (9-13).
2.3.4. Experiment 3: Equal Rewards Condition
To test for delay perception and response perseveration, the reward for each lever was equalized (1 food pellet for either choice) while the delays remained the same as in Experiment 2 (thus rendering the no delay lever the optimal choice in all blocks). Rats were run in this condition for 10 sessions.
2.3.5. Experiment 4: No-Delay Condition
To examine reward magnitude perception and to further test for response perseveration, the reward magnitudes were restored to their initial conditions (1 food pellet vs. 4 food pellets) and the delays preceding the large reward were eliminated, (thus rendering the large reward lever the optimal choice in all blocks). Rats were run in this condition for 10 sessions.
2.3.6. Experiment 5: Fixed Ratio Responding
As a final test of motivation to obtain food, rats were tested for 5 sessions in a different set of 4 behavioral test chambers with dimensions identical to those used in the delay discounting task but with only a single response lever fixed in place 3.5 cm above the food trough. In each 30 minute session, presses on this lever resulted in delivery of a single food pellet on various fixed ratio (FR) schedules (FR1, FR3, FR10, FR20, FR40, one schedule per session).
2.4 Data Analysis
Raw data files were exported from Graphic State software and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, Dept. of Anatomy and Neurobiology, Virginia Commonwealth University). Statistical analyses were conducted in SPSS 12.0. In Experiments 1-4, data were averaged across the final 5 sessions in each experiment [10,43], and these averages were analyzed using two-factor repeated measures ANOVAs (age group × delay duration). Additional analyses (three-factor repeated measures ANOVAS; age group × delay duration × session) were performed to determine whether young adult and aged rats differed in their rates of adaptation to the changes in task contingencies between experiments (i.e. – to assess differences in perseverative behavior). Data from shaping sessions and fixed ratio responding in Experiment 5 were analyzed using independent samples t-tests. In all cases, p values less than .05 were considered significant.
3. Results
3.1. Shaping
There were no differences between young adult and aged rats in the number of sessions required to complete either of the phases of shaping (lever press or nosepoke shaping, ts < 0.80, ps > .48), indicating that both groups were able to acquire the task procedures at a similar rate (young adult mean: 13.25, SEM = 2.66; aged mean: 14.25, SEM = 2.09). Following shaping, rats were tested for a total of 78 days in Experiments 1-5.
3.2. Experiment 1
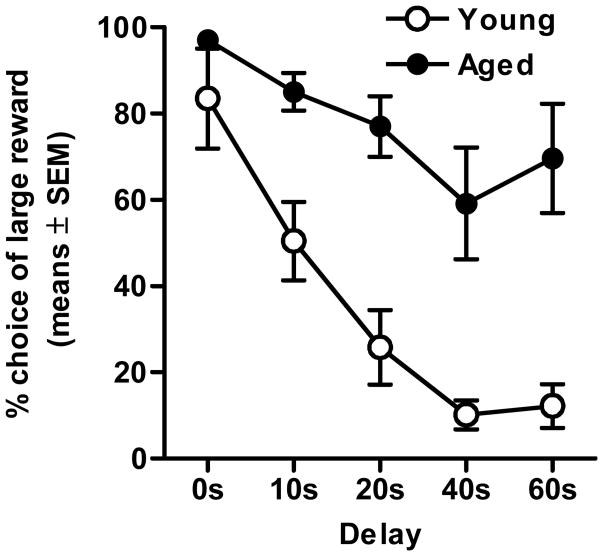
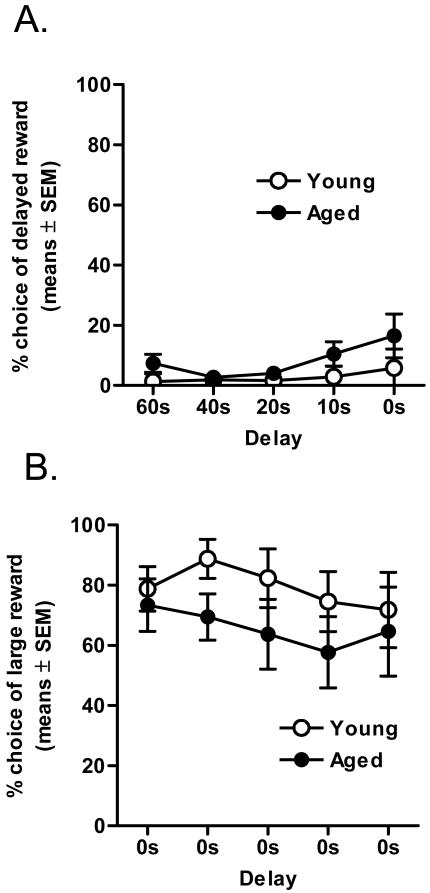
As shown in Figure 1, a two-factor ANOVA (delay × age) revealed that all rats decreased their preference for the large reward as the delay to large reward delivery increased (F(4, 56) = 24.16, p <.01). Most importantly, however, there was a robust and significant main effect of age (F(1, 14) = 20.88, p <.01), as well as a significant interaction between delay and age (F(4, 56) = 3.83, p <.01). These data demonstrate that discounting of delayed rewards is dramatically attenuated in aged rats compared to their young adult cohorts.
Figure 1. Delay discounting in young adult and aged rats (Experiment 1).
All rats discounted the value of the large reward (as shown by decreased preference for the large reward) as delays increased (p <.01). However, aged rats showed less discounting than young adults (p <.01).
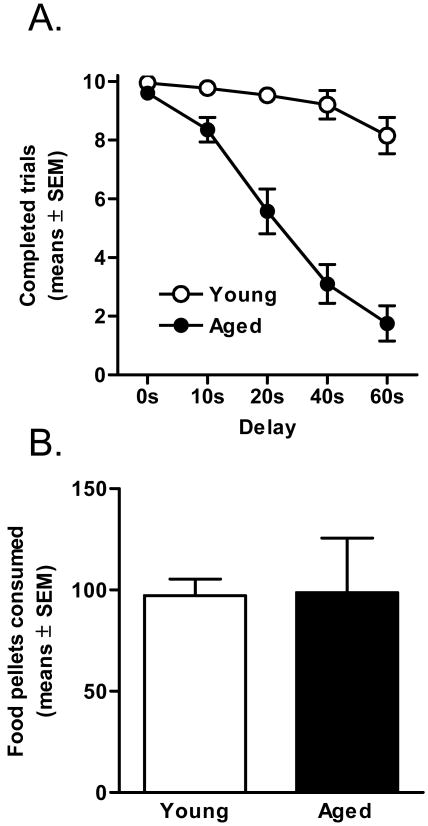
It should be noted that aged rats omitted significantly more trials than young adult cohorts, particularly in the trial blocks with the longest delays, which occurred later in the session. Analysis of the number of completed trials (defined as trials with a response on either lever) across the session using a two-factor ANOVA (delay duration × age group) confirmed both a main effect of age (F(1, 14) = 49.50, p <.01) and an interaction between the factors of age and delay (F(4, 56) = 68.70, p <.01), such that aged rats completed significantly fewer trials than young adult cohorts, and this effect became larger across successive trial blocks (Figure 2A). This difference could be due to the order of presentation of the delays, increased fatigue, or faster satiation in the aged rats. In order to assess satiation, the amount of total food consumed across trials was assessed. Indeed, despite completing fewer trials, because aged rats consistently chose the large reward more often than their young adult cohorts, both age groups consumed comparable amounts of food across sessions (t(14) = .16, p = .91; Figure 2B). To determine the influence of the order of delay presentations and fatigue on the age-related difference in discounting, in Experiment 2, the same subjects were tested further on the same task with the order of the delay presentations reversed.
Figure 2. Total responses and food consumed (Experiment 1).
(A) Aged rats responded less frequently than young adults in later blocks of trials (p <.01). (B) Despite this difference in omitted trials, however, young adult and aged rats consumed equal amounts of food per session.
3.3. Experiment 2
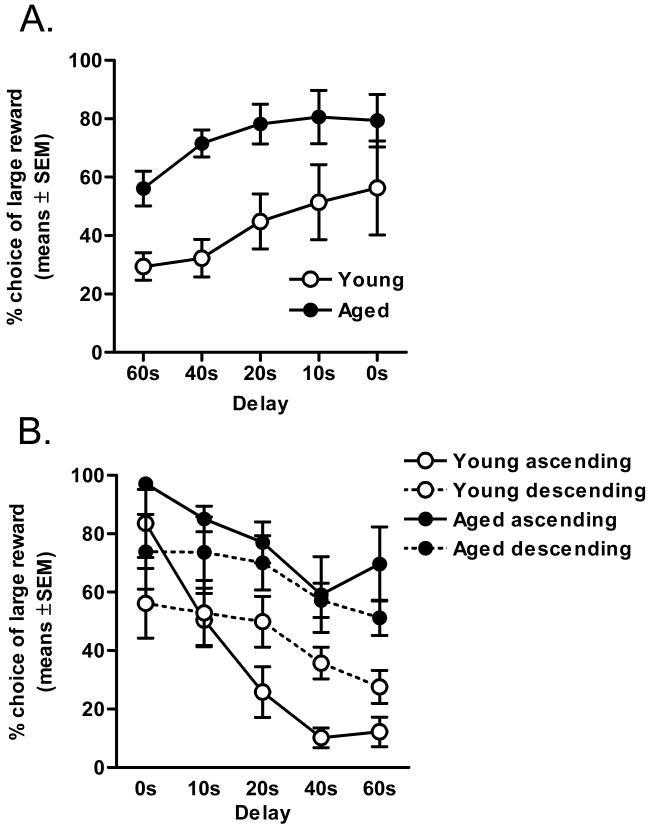
In this condition, the order of delay presentation was reversed, such that the longest delay to large reward delivery (60 s) was presented first, with subsequent delays to large reward delivery decreasing in order to 0 s. Rats in both groups reached stable responding in this phase of testing by day nine, and the subsequent five sessions (9-13) were analyzed. In agreement with the findings in Experiment 1, both age groups showed a decreased preference for the large reward lever with longer delays to large reward delivery (two-factor ANOVA (delay duration × age group); F(4, 52) = 6.06, p < .01; Figure 3A). Importantly, there was still a significant main effect of age (F (1, 13) = 7.64 p < .05), such that aged rats chose the large delayed reward more often than young adult cohorts (although the interaction between the factors of delay and age was no longer significant, F(4, 52) = .54, p = .71). Further repeated measures ANOVAs comparing performance on the ascending (Experiment 1) and descending (Experiment 2) order of delays within each age group (Figure 3B) revealed no main effect of age on choice behavior across these conditions (ps > .29). Together, these data strongly indicate that the order of delay presentations was not solely responsible for the age-related attenuation of delay discounting.
Figure 3. Delay discounting in young adult and aged rats with order of delays reversed (Experiment 2).
(A) Aged rats continued to discount the value of large rewards to a lesser degree than young adult cohorts when the order of delay presentations was reversed (p <.05). Thus, the effects of age on delay discounting in Experiment 1 were not due to fewer responses in aged rats at longer delays. (B) Data from Figures 1 and 2A are re-plotted using the same X axis to facilitate direct comparison of performance with ascending and descending orders of delay presentation.
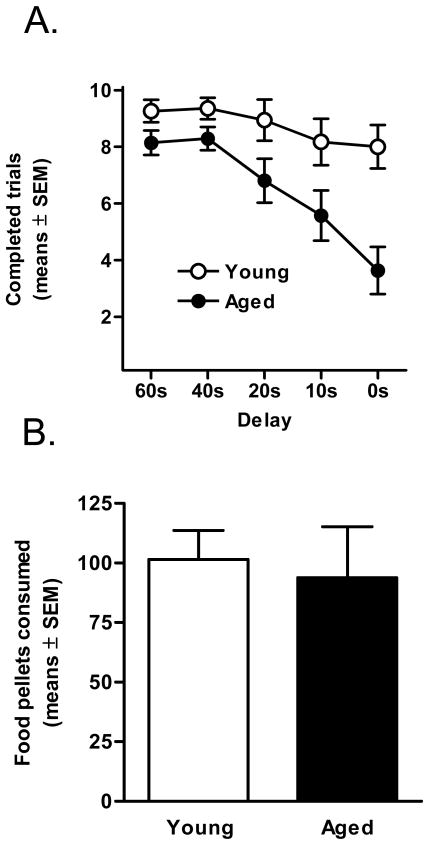
Analyses of the number of completed trials in each trial block revealed the same pattern of results observed with the ascending order of delay presentations in Experiment 1, such that aged rats increased their number of omitted trials in blocks later in the sessions, which in this experiment were the blocks with the shortest delays (Figure 4A). A two-factor ANOVA (delay duration × age group) revealed a main effect of age (F(1, 13) = 8.05 p <.05) and an interaction between delay and age (F(4, 54) = 5.39, p < .01). These data suggest that satiation (or possibly fatigue) in the aged rats was likely a significant factor in the low response rates of aged subjects in later blocks of trials. Indeed, as in Experiment 1, food consumption across sessions was equivalent between age groups when the delays were reversed (t(13) = .51, p = .62; Figure 4B). Most importantly, the observation that aged rats preferentially chose the large delayed reward more often than young adult rats in both conditions (ascending and descending order of delay presentations) indicates that the effects of age on delay discounting are independent of the order of presentation of the delays and number of completed trials.
Figure 4. Total responses and food consumed with order of delays reversed (Experiment 2).
(A) Similar to Figure 2, aged rats responded less as the session progressed (p <.05), even though the order of delay presentations was reversed. This indicated that the increase in trial omissions in aged rats was not a function of the order of delay presentations. (B) Young adult and aged rats consumed equivalent amounts of food per session.
3.4. Experiment 3
To determine whether aged rats were less sensitive than young adult cohorts to the aversive consequences of delayed reward delivery, the task contingencies were modified such that both the immediate and delayed rewards consisted of only a single food pellet. Rats performed under these contingencies for 10 sessions and stable responding was reached by session 6 for both groups. In both age groups, choice of the delayed reward dropped to near-zero levels (Figure 5A). Analysis of the mean percentage of choices of the delayed reward in sessions 6-10 using a two-factor ANOVA (delay duration × age group) revealed neither a main effect nor any interactions involving age (Fs < 1.94, p > .19). The data strongly suggest that the ability of aged rats to perceive and respond to delays was comparable to that of young adult cohorts.
Figure 5. Tests of delay and reward magnitude perception (Experiments 3 and 4).
(A) Aged rats were able to detect and respond appropriately to the delays to the same degree as young adult rats when the delays were maintained but the reward magnitudes made equal. (B) Aged rats were able to detect and respond appropriately to the different reward magnitudes to the same degree as young adult rats when the reward magnitudes were maintained but the delays made equal.
3.5. Experiment 4
To determine whether aged rats were differentially sensitive to the different reward magnitudes compared to young adult cohorts, the task contingencies were modified such that the reward magnitudes were returned to their original condition (1 vs. 4 food pellets), but the delays to the large reward were eliminated (i.e. - a zero delay was imposed across all trial blocks). Rats performed under these contingencies for 10 sessions and stable responding was obtained by session 6. Subjects in both age groups switched to responding on the lever with the larger reward (Figure 5B). Analysis of the mean percentage of choices of the large reward lever in sessions 6-10 using a two-factor ANOVA (delay duration × age group) revealed neither a main effect nor any interactions involving age (Fs < 1.46, ps > .23). These data strongly suggest that young adult and aged rats in this study were similarly sensitive to the different reward magnitudes used in the task.
3.6. Analyses of perseverative behavior in young adult and aged rats
To determine whether age-related differences in delay discounting could be accounted for by perseverative responding on the large reward lever, a number of additional analyses were conducted. First, a three-factor ANOVA (delay duration × age group × session) conducted across all 13 sessions of Experiment 2 (in which the order of delay presentations was reversed) revealed no interactions between age and session (Fs < 1.32, ps > .21). These data suggest that aged rats had no more difficulty than young adult rats in altering their choice pattern from that used in Experiment 1. Moreover, in Experiment 3, when reward magnitudes were made equivalent, aged rats switched their responding to the lever that produced the immediate reward at a rate comparable to young adult cohorts (no interactions between age and session (Fs < 1.10, ps > .30) in a three-factor ANOVA (delay duration × age group × session) conducted across all 10 sessions of the equal-reward condition). Finally, in Experiment 4, when delays were made equivalent across trials, aged rats switched their responding to the lever that produced the large food reward at a rate comparable to young adult rats (no interactions between age and session (Fs < 1.69, ps > .10); three-factor ANOVA (delay duration × age group × session) conducted across all 10 sessions of equal-delay condition). In sum, aged and young adult rats appeared comparable in their ability to alter their choice pattern in accord with changes in task contingencies, arguing against increased perseveration being a primary factor in the marked attenuation of delay discounting in aged rats.
3.7. Experiment 5
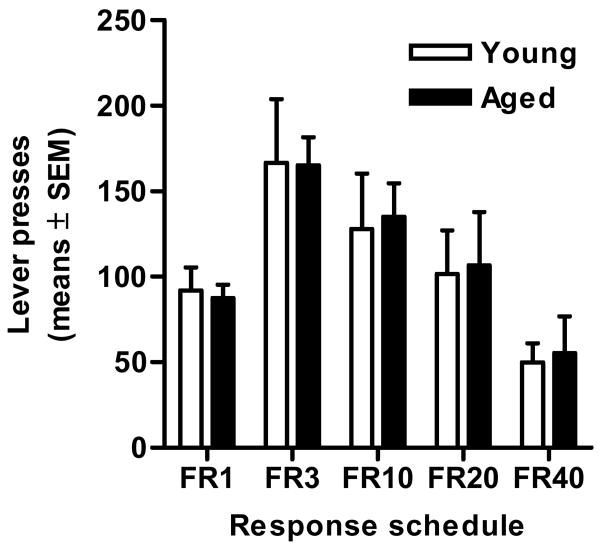
To determine general motivation to work for food (particularly under conditions of high response ratios, which are necessarily correlated with delayed reward delivery), rats were placed in a new testing environment and given the opportunity to press a single lever (located above the food trough) to obtain food pellets. Rats were initially tested under a fixed ratio 1 (FR1) schedule, on which 1 lever press was required for food delivery, and the response ratio requirement was increased in subsequent daily sessions. Comparisons of the total number of lever presses in each session in young adult and aged rats revealed no differences between groups on performance under any response ratio (ts < 0.30, ps > .79), demonstrating that young adult and aged rats were similarly able and motivated to perform the lever-pressing responses involved in the delay discounting task (Figure 6).
Figure 6. Fixed ratio responding (Experiment 5).
Aged rats performed comparably to young adult rats across a range of free-operant fixed ratio schedules of responding for single food pellets, suggesting that aged and young adult rats were similarly motivated to respond for and obtain the food reward used in the delay discounting task.
4. Discussion
The results of these experiments demonstrate that aged Fischer 344 rats show significantly attenuated discounting of delayed rewards compared to young adult cohorts (i.e. – aged rats prefer large delayed over small immediate rewards to a greater extent than young adult rats). This attenuated delay discounting was not likely due to increased perseverative responding or age-related changes in motivation or perception of reward or delay magnitude, as aged rats performed comparably with young adults on several control tasks. This difference was also not likely due to especially steep delay discounting in the young adult rats. Although adolescents are reported to discount delayed rewards more steeply than adults, the young adult rats began these experiments at 6 months of age, which is well beyond the age at which rats are considered to be sexually mature [6,22]. These findings suggest that age-related changes in decision making observed in humans are not solely due to experiential and life-history factors, and that such changes can be effectively modeled in rodents [14, 21, 22, 28].
The main finding from this series of experiments (depicted in Figure 1) is that in comparison to young adult rats, aged rats reliably and robustly prefer a large, delayed reward over a smaller, immediate reward. Despite this clear age difference, of some initial concern was the fact that aged rats made fewer overall responses than young adult rats (i.e. – had more omissions), particularly during the later trial blocks on which the greatest age differences were observed. This increase in omissions over the course of the sessions could be due to satiation, fatigue, or frustration with the long delays, which might have skewed the results to produce the observed difference in choice behavior. Two additional analyses were conducted to specifically address these possibilities. First, total food consumption per session was assessed for each group and found to be identical. Thus, although satiation earlier in the session may have caused the increased omissions in aged rats, such satiation seemed to be a consequence, rather than a cause of their choice behavior, as aged rats' satiation threshold was similar to that of young adults (at least to the extent examined here). Second, in Experiment 2 the order of presentation of the delays until large reward delivery was reversed such that the block of trials with the longest delay in Experiment 1 (during which aged rats had the most omissions) was presented first (Figure 3). Both young adult and aged rats displayed the same patterns of omitted trials during these sessions as in Experiment 1 (i.e. – increasing numbers of omissions as the session progressed), although in this reversed delay order condition, the majority of omissions occurred in blocks with the shortest delay. These data strongly suggest that the omissions observed at the longest delay in Experiment 1 were unrelated to the delay duration. Most importantly, the same age differences in delay discounting were observed when the order of delays was reversed, indicating that these differences were not an artifact of trial omissions. The failure of both young adult and aged rats to reach the high levels of % choice of the large reward at the 0 s delay during Experiment 2 was likely due to some perseverative choice of the small reward lever in both groups, and is consistent with the findings of Cardinal et al. [10] under these reversal conditions.
Aging has been associated with changes in time perception, in that subjective time appears to pass more slowly for aged compared to young adult humans [4]. Similarly, aged rats tested in a peak interval procedure show judgments of 20 and 40 s time intervals that are longer than those of young adult rats [31,33]. This subjectively slower passage of time associated with aging would be predicted to bias aged rats away from choice of the delayed reward [53]. However, the aged rats in the present experiments chose the delayed reward more frequently than young adult rats, suggesting that reported alterations in time perception in aging do not account for the altered discounting of delayed rewards. Further evidence against age-related differences in time perception is provided by the results of Experiment 3, in which aged rats were able to detect and respond appropriately to the delays to the same degree as young adult rats when the delays were maintained but the reward sizes made equal (i.e. – they consistently chose the non-delayed over the delayed reward). This control procedure suggests that gross insensitivity to delays does not account for the attenuated delay discounting in aged rats, although more subtle impairments related to time perception are possible. Indeed, the fact that young adult and aged rats differed in delay discounting task performance suggests that some aspect of delay processing differed across ages. Interestingly, Niv and colleagues [35] have suggested that tonic dopamine levels in striatum encode the subjective value of time. By this hypothesis, decreased tonic dopamine levels would result in time being valued less, leading to a reduction in the subjective costs of choosing the large delayed reward. In support of this idea, reductions in dopaminergic markers have been reported in aged rats, suggesting that age-related alterations in dopamine signaling are one possible mechanism that could contribute to the observed results [7].
Putative The results of several control experiments also suggest that alterations in reward perception and/or motivation do not account for the effects of aging on delay discounting. Although delay discounting is regulated by reward magnitude, level of hunger [5,54], and food metabolism, which can be altered significantly in aging [24,45], when the delays to large reward delivery were eliminated (Experiment 4), aged rats chose the large reward to the same extent as young adults. These data indicate comparable perception and reaction to these differences in reward magnitude in the two groups. Moreover, in Experiment 5, young adult and aged rats had equivalent levels of instrumental responding under various fixed ratio schedules, demonstrating equivalent motivation to work for and obtain food rewards, even under high ratios of responding which imposed substantial delays between initiation of lever-pressing and food delivery.
In the delay discounting task, learning about the relationship between the large reward lever and the delayed reward with which it is associated, and subsequent choice of that lever, would be presumed to require maintenance of some representation of the large reward outcome across the delay preceding its delivery [23]. Indeed, maintenance of such outcome representations across delays can be impaired in aging [27,32]. However, impairments in the ability to learn and utilize associations between the large reward lever and the delayed reward itself would be expected to bias responding away from choice of the large reward. Because aged rats chose the large delayed reward more often than young adult cohorts, such impairments do not likely account for the attenuated delay discounting reported here.
Performance on this delay discounting task is mediated by a network of limbic-striatal brain structures, including orbitofrontal cortex (OFC), basolateral amygdala, ventral striatum, and subthalamic nucleus, as well as their dopaminergic and serotonergic innervation [8,9,48,50,51]. In particular, lesions of OFC can decrease discounting of delayed rewards in the task used here (although this effect may depend on pre-lesion training on the task [34,50]), and OFC is compromised with age in both rats and humans [12,29,41,42]. One behavioral function ascribed to OFC is assignment of motivational value to rewards under conditions in which this value changes rapidly [19,25,38,40]. Because “normal” performance in the delay discounting task involves tracking the changing reward value as the delays change across a session and modifying responses accordingly, age-related impairments in these abilities could result in increased choice of the large reward at longer delays (indeed, some evidence suggests that a subpopulation of aged humans is impaired in tracking changes in reward value [13,14,18,28]). Note, however, that the pattern of results in the aged rats is not consistent with simple perseverative responding on the large reward lever. There was no evidence for increased perseverative responding in aged compared to young adult rats after the order of presentation of the delays was reversed (i.e. – the slopes of their performance curves are similar in Figure 3B), and young adult and aged rats modified their choice behavior at similar rates in response to each change in the task contingencies. It should be noted, however, that although attenuated delay discounting in the aged rats was clearly advantageous under the task contingencies employed here (i.e. - it resulted in more food delivery earlier in the session), it could likely prove maladaptive in other situations, particularly when behavioral flexibility provides superior advantage [41].
The results of these experiments provide strong evidence that discounting of delayed rewards is attenuated in aging. Several control procedures suggest that this attenuated discounting is not due to age-related alterations in time perception, motivation, or perseveration. These findings are consistent with the idea that aging is associated with increased ability to delay gratification (i.e. – “good things come to those who wait”). The fact that these findings were obtained in laboratory rats (which, unlike humans, have limited prior opportunities to learn about the benefits of delayed gratification) suggests that attenuated delay discounting results not solely from experiential factors, but also (or instead) from fundamental age-related neurobiological changes. Of course, it is not possible even in animal subjects to rule out entirely the influence of prior experience on decision-making, as even the controlled and relatively impoverished environment of laboratory housing could conceivably provide opportunities for learning to delay gratification. Nevertheless, the results clearly show that decision-making tasks used in humans can be effectively and robustly modeled in aged rodents, which will enable future investigation of the neural basis of altered decision-making across the lifespan.
Acknowledgments
We thank Atasi Bhavsar and Deepa Ramamurthi for technical assistance. Supported by: AG029421 (JLB & BS), DA023331 (NWS), NS059324 (CLL), and MH65728 (IAM) from the National Institutes of Health.
Footnotes
Disclosure Statement: There are no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 2.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 3.Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2007.08.004. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block RA, Zakay D, Hancock PA. Human aging and duration judgments: a meta-analytic review. Psychology and Aging. 1998;1998:584–96. doi: 10.1037//0882-7974.13.4.584. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw CM, Szabadi E. Choice between delayed reinforcers in a discrete-trials schedule: the effect of deprivation level. The Quarterly Journal of Experimental Psychology. 1992;44:1–6. doi: 10.1080/02724999208250599. [DOI] [PubMed] [Google Scholar]
- 6.Brenhouse HC, Sonntag KC, Andersen SL. Transient d1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. The Journal of Neuroscience. 2008;28:2375–82. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burwell RD, Lawler CP, Gallagher M. Mesostriatal dopamine markers in aged Long-Evans rats with sensorimotor impairment. Neurobiology of Aging. 1995;16(2):175–86. doi: 10.1016/0197-4580(94)00157-x. [DOI] [PubMed] [Google Scholar]
- 8.Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Networks. 2006;19:1277–301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Cardinal RN, Pennicot DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 10.Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signaled and unsignaled delayed reinforcement in rats. psychopharmacology. 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- 11.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 12.Convit A, Wolf OT, de Leon MJ, Patalinjug M, Caraos C, Scherer A, Saint Louis LA, Cancro R. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Research. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- 13.Deakin JF, Aitken M, Robbins TW, Sahakian BJ. Risk taking during decision-making in normal volunteers changes with age. Journal of the International Neuropsychological Society. 2004;10:590–8. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- 14.Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2003;36:449–58. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 17.Federal Interagency Forum on Aging-Related Statistics. [September 25, 2005];2005 September 25, 2005. Older Americans 2004: Key Indicators of Well-Being. < http://www.agingstats.gov/chartbook2004/population.html>.
- 18.Fein G, McGillivray S, Finn P. Older adults make less advantageous decisions than younger adults: cognitive and psychological correlates. Journal of the International Neuropsychological Society. 2007;13:480–9. doi: 10.1017/S135561770707052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fellows LK. The role of orbitofrontal cortex in decision making: A component process account. Annals of the New York Academy of Sciences. 2007 doi: 10.1196/annals.1401.023. EPub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 20.Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiology of Aging. 1995;16(2):149–60. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 21.Green L, Fry AF, Myerson J. Discounting of delayed rewards: a life-span comparison. Psychological Science. 1994;5:33–6. [Google Scholar]
- 22.Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal Discounting in choice between delayed rewards: the role of age and income. Psychology and Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- 23.Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology Learning, Memory, and Cognition. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- 24.Kiang-Ulrich M, Horvath SM. Age-related metabolic modifications in male F344 rats. Experimental Aging Research. 1984;10:89–93. doi: 10.1080/03610738408258549. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of Learning and Memory. 2005;83:125–33. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby KN, Petry NM. Heroin and cocaine abusers of higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 27.Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Age-related effects in eyeblink conditioning in the F344 × BN F1 hybrid rat. Neurobiology of Aging. 2001;22:1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 28.Lamar M, Resnick SM. Aging and prefrontal functions: dissociating orbitofrontal and dorsolateral issues. Neurobiology of Aging. 2004;25:553–8. doi: 10.1016/j.neurobiolaging.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Lamar M, Yousem DM, Resnick SM. Age differences in orbitofrontal activation: an fMRI investigation of delayed match and nonmatch to sample. Neuroimage. 2004;21:1368–76. doi: 10.1016/j.neuroimage.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 30.LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiology of Aging. 2007;28(6):928–36. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Lejeune H, Ferrara A, Soffie M, B M, Weardon JH. Peak procedure performance in young adult and aged rats: acquisition and adaptation to a changing temporal criterion. The Quarterly Journal of Experimental Psychology. 1998;51:193–217. doi: 10.1080/713932681. [DOI] [PubMed] [Google Scholar]
- 32.McEchron MD, Cheng AY, Gilmartin MR. Trace fear conditioning is reduced in the aging rat. Neurobiology of Learning and Memory. 2004;82:71–6. doi: 10.1016/j.nlm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Meck WH, Church RM, Wenk GL. Arginine vasopressin innoculates against age-related increases in sodium-dependent high affinity choline uptake and discrepancies in the content of temporal memory. European Journal of Pharmacology. 1986;130:327–31. doi: 10.1016/0014-2999(86)90287-6. [DOI] [PubMed] [Google Scholar]
- 34.Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–8. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 35.Niv Y, Daw N, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191(3):507–20. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- 36.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 37.Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. Journal of Abnormal Psychology. 2001;110:482–7. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- 38.Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. The Journal of Neuroscience. 2003;23:11078–84. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rachlin H, Green L. Commitment, choice, and self-control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roesch MR, Calu DJ, Burke KA, Schoenbaum G. Should I stay or should I go? Transformation of time-discounted rewards in orbitofrontal cortex and associated brain circuits. Annals of the New York Academy of Sciences. 2007;1104:21–34. doi: 10.1196/annals.1390.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenbaum G, Nugent SL, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiology of Aging. 2002;13:885–90. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- 42.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. The Journal of Neurophysiology. 2006;95:1509–17. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long term increases in impulsive choice. Behavioral Neuroscience. 2007;121:543–9. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sozou PD, Seymour RM. Augmented discounting: interaction between ageing and time-preference behaviour. Proceedings of the Royal Society of London Series B: Biological Sciences. 2003;270:1047–53. doi: 10.1098/rspb.2003.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speakman JR, Selman C, McLaren JS, Harper EJ. Living fast, dying when? The link between aging energetics. The Journal of Nutrition. 2002;132:1583S–97S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- 46.van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biological Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 47.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 48.Winstanley CA, Baunez C, Theobald DEH, Robbins TW. Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. European Journal of Neuroscience. 2005;21:3107–16. doi: 10.1111/j.1460-9568.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- 49.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006;26:379–95. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of Neuroscience. 2004;24:4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winstanley CA, Theobald DEH, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cerebral Cortex. 2006;16:106–14. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- 52.Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between Serotonin and Dopamine in the Control of Impulsive Choice in Rats: Therapeutic Implications for Impulse Control Disorders. Neuropsychopharmacology. 2005;30(4):669–82. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- 53.Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends in Cognitive Sciences. 2008;12(1):7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Wogar MA, Bradshaw CM, Szabadi E. Choice between delayed reinforcers in an adjusting-delay schedule: the effects of absolute reinforcer size and deprivation level. Quarterly Journal of Experimental Psychology B, Comparative and Physiological Psychology. 1992;45:1–13. [PubMed] [Google Scholar]
- 55.Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. Journal of Gerontology. 1982;37:130–41. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]