FIG. 4. Changes of site-specific histone modifications in the soluble and insoluble chromatin in apoptotic thymocytes.
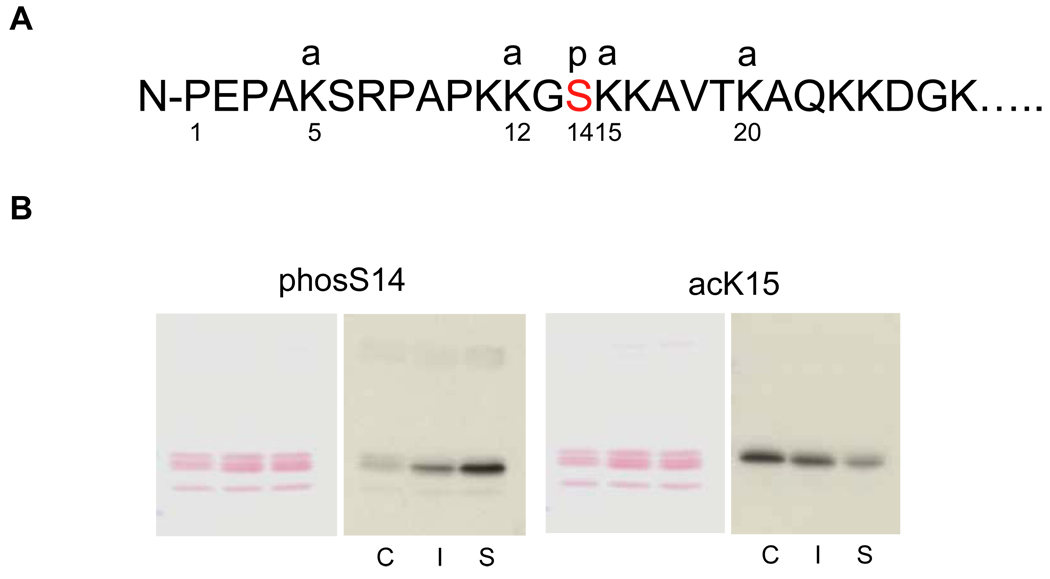
(A) The sites of H2B modifications at the N-terminal tail. Four sites are known to be acetylated at 5, 12, 15 and 20th residues of lysine, whereas a phosphorylation occurs at the 14th serine in the middle of an acetylation group. a: acetylation, p: phosphorylation.
(B) Acetylation and phosphorylation of H2B in the soluble and insoluble chromatin. Soluble and insoluble chromatin was prepared from dexamethasone treated thymocytes and histones were extracted. The histones were blotted against antibodies for site-specific modifications (phosS14 and acetyl K15) (right panel). Proteins were stained with Ponceau S (left panel). C. Control chromatin, I: Insoluble chromatin from dexamethasone treated thymocytes. S: Soluble chromatin from dexamethasone treated thymocytes. The data shown is representative of an N=4.
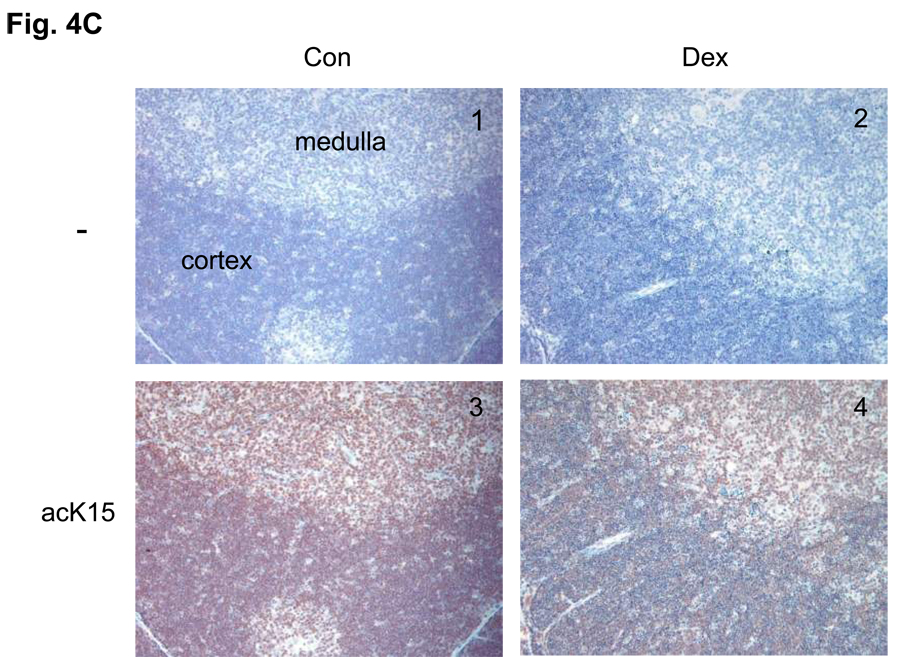
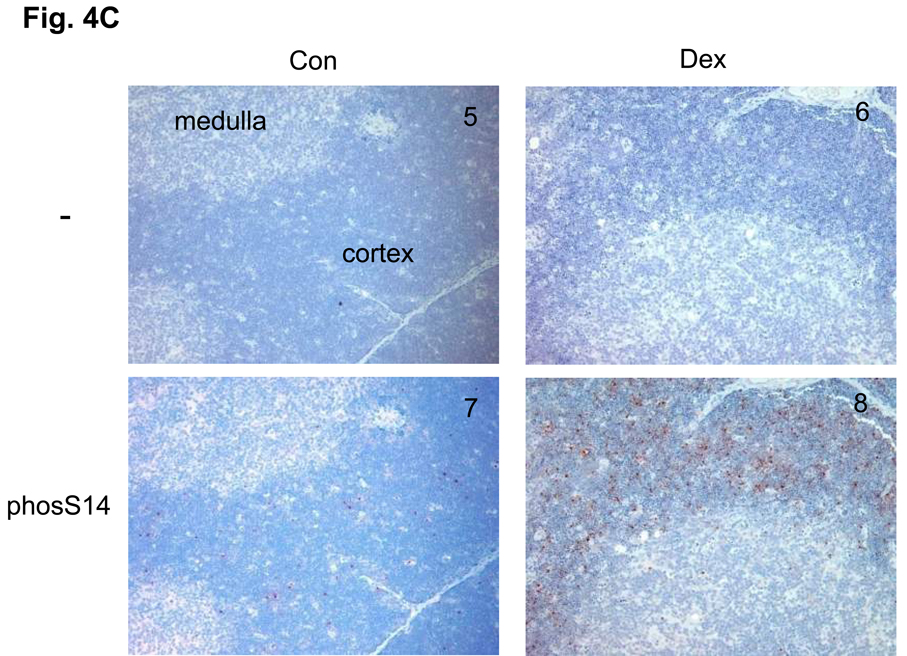
(C) Immunohistochemical analysis of phosS14 and acK15 in thymus tissues. Rat thymus was dissected and the tissues were treated with or without antibodies for histone modifications and stained with horse radish peroxidase. Brown color was observed as a positive reaction of a specific antibody. Pictures from 1 to 4 are a series of immunohistochemical analysis against acK15 and from 5 to 8 are for the analysis of phosS14. 1 and 5: Thymus section from the control animal treated without antibody. 2 and 6: Thymus section from the dexamethasone treated animal and treated without antibody. 3 and 7: Control section with the antibody. 4 and 8: Dexamethasone section with antibody.
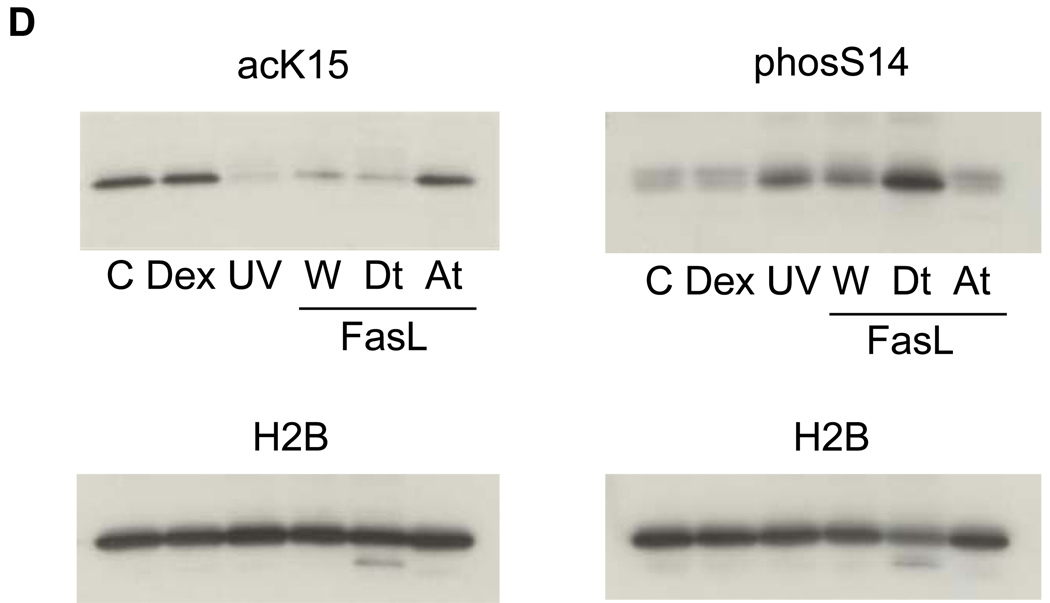
(D) A switch of histone modifications between acK15 and phosS14 in H2B of apoptotic HTC cells. Apoptosis was induced in HTC cells by UV-C or Fas ligand. Attached and detached cells were separated by a gentle shaking from glass substratum. Histones were extracted and analyzed by a western blotting against anti-phosS14. C, Control; Dex, dexamethasone; UV, whole cells treated with UV-C; W, whole cells treated with Fas ligand, At and Dt; attached and detached whole cells, respectively from Fas ligand treated cells. Amount of each sample was normalized by H2B detected with an anti-H2B antibody. The data shown is representative of an N=3.