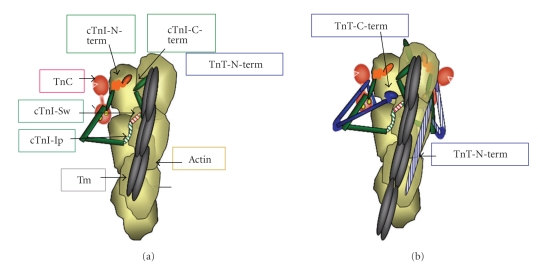
Figure 1.
Structure of a patch of cardiac thin filament demonstrating the position of troponin components with tropomyosin (Tm) in the “off” state. (a) Thin filament containing, only troponin I (cTnI) and troponin C (cTnC). cTnC is shown as a dumbbell shaped protein with the N-lobe containing a single regulatory Ca-binding site. cTnI is shown tethered to actin on an actin strand by an inhibitory peptide (Ip) and a second actin binding region flanking a switch peptide (Sw), which interacts with cTnC upon Ca-activation. The distal C-terminal end of cTnI drapes across azimuthal actins and may interact with Tm. cTnI has a unique stretch of N-terminal amino acids, which contain phosphorylation sites at S23, S24. The N-peptide interacts with the N-lobe of cTnC, but is released upon phosphorylation and may react with the Sw or Ip of cTnI. (b) Thin filament contains the complete Tn complex. Note that the Ip of cTnI binds to one actin strand, whereas the N-terminal tail of troponin T (cTnT) interacts with Tm on the adjacent actin strand. The consequence is that Tm is wedged and held in a blocking position.