Figure 1.
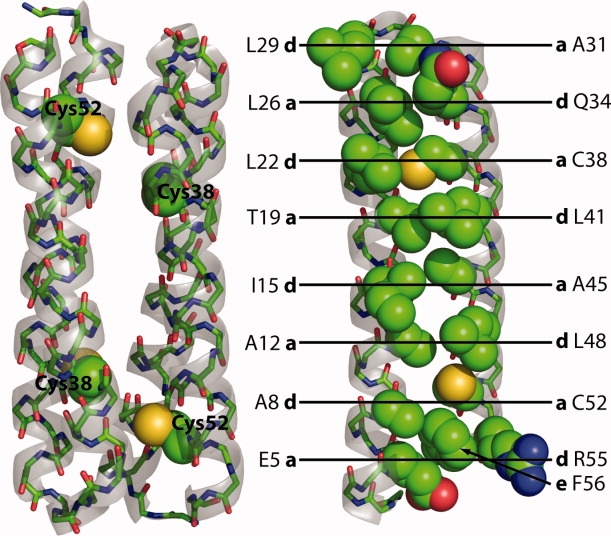
The structure of wild-type Rop. Rop is an antiparallel homodimer of 63 amino acid monomers, placing the reduced Cys38 and Cy52 residues near each other across the dimer interface. At right, in the monomer, residues in the hydrophobic core of Rop are shown as spheres and labeled with their position (a, d, or e) in the heptad repeat. Note that in the last repeat, R55 (d) is mostly exposed and F56 (e) instead packs into the core. Rendered from 1ROP with PyMOL (Delano Scientific).
