Figure 6.
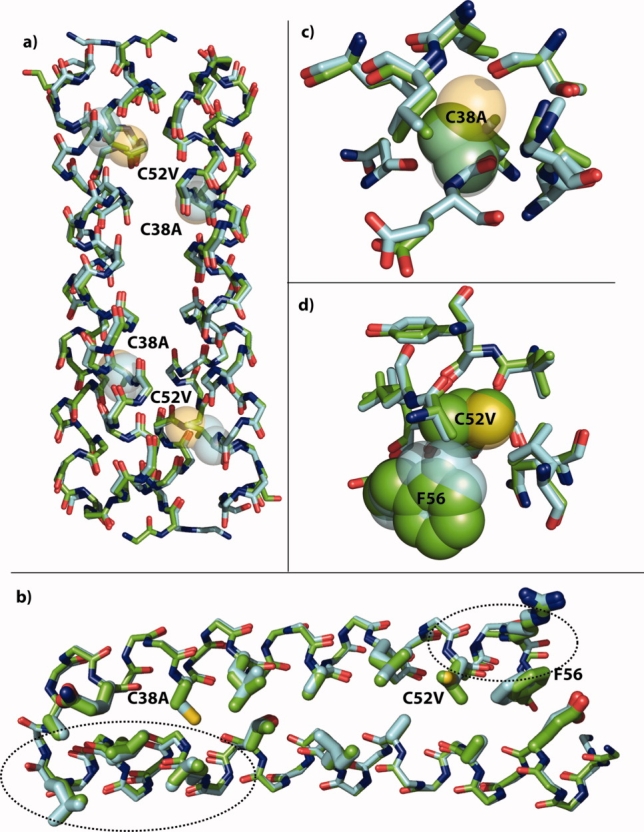
X-ray crystal structure of Ala/Val Rop. (a) Overlay of the backbones of the symmetry-derived dimers of wild-type Rop (cyan carbons) and Ala/Val Rop (green carbons). The positions of the C38A and C52V mutations are shown as 50% transparent spheres for the wild-type Cys side chains. There is little change in the overall structure. (b) Overlay of the monomer backbones with core residues rendered as thick sticks. The backbone in helix 1 opposite C38A has moved slightly toward position 38. Near C52V, a small movement of the backbone has displaced F56 toward the open end of the hairpin. (c) Resides with atoms within 5 Å of the Cys38 sulfur are nearly unchanged except for the slight backbone shift toward the site of mutation. (d) It is evident that Val38 would have clashed with position of Phe56 in the wild-type, and it is displaced slightly by movement of the backbone. In (c) and (d), C38, C52, and F56 are shown in 50% transparent spheres with cyan carbons in the wild-type, and A38, V52, and F56 are shown in solid spheres with green carbons in Ala/Val. Rendered with PyMOL from 1ROP and the structure solved here, 3K79.
