Abstract
Boosting BCG-primed mice with a recombinant adenovirus expressing M. tuberculosis antigen 85A by different routes has very different effects on protection against aerosol challenge with M. tuberculosis. Mice boosted intradermally make very strong splenic CD4 and CD8 Th1 cytokine responses to antigen 85A, but show no change in lung mycobacterial burden over BCG primed animals. In contrast intranasally boosted mice show greatly reduced mycobacterial burden and make a much weaker splenic response but a very strong lung CD4 and CD8 response to antigen 85A and an increased response to PPD. This is associated with the presence in the lung of multifunctional T cells, with high median fluorescence intensities and integrated median fluorescence intensities for IFNγ, IL-2 and TNF. In contrast, mice immunized with BCG alone have few antigen-specific cells in the lung and a low proportion of multi-functional cells although individual cells have high median fluorescence intensities. Successful immunization regimes appear to induce antigen-specific cells with abundant intracellular cytokine staining.
Keywords: rodent, T cells, bacterial, lung, vaccination
Introduction
M. tuberculosis infection is a global epidemic with 1.6 million deaths per year (1). The emergence of HIV-associated mycobacterial infections and increasing frequency of multi-drug resistant isolates reinforces the need to develop new control strategies. Although BCG confers a degree of protection against disseminated disease in the very young, protection against pulmonary disease in older age groups is poor.
Because BCG provides partial protection, an attractive strategy is to develop vaccines that can be used as boosters following BCG primary immunization. Attempts to boost CD4 immunity above the level induced by BCG alone have had variable success. For example, mice immunized with recombinant antigen 85B protein are very poorly protected although they develop a splenic CD4 T cell population producing large amounts of IFNγ (2, 3). In contrast, intranasal BCG priming followed by an intranasal boost with recombinant modified vaccinia Ankara expressing the abundant and immunogenic M. tuberculosis antigen 85A (MVA85A), generates increased protection against aerosol challenge, associated with increased numbers of CD4 85A-specific IFNγ-secreting T cells in lung lymph nodes (4).
IFNγ has been shown to be necessary but not sufficient for protection against M. tuberculosis. Th1 CD4 cells are the main producers of cytokines following BCG immunization and therefore the frequency of IFNγ producing cells has been widely used as a correlate of protection against M. tuberculosis. However, recent data from mice and cattle, show that measurement of spleen or blood IFNγ producing CD4 cells does not correlate with protection (3, 5, 6). Furthermore although BCG immunization stimulates long-lasting, predominantly Th1 protective immunity in both mice and humans, the use of CD4 or CD8 deficient mice indicates that CD8 cells can also be protective (7, 8). Increased protection over BCG, can be achieved also by immunization with a proapoptotic mutant of M. tuberculosis or prime boost regimes using recombinant Adenovirus containing antigen 85A (Ad85A) (9-12). These regimes generate strong lung CD8 IFNγ responses. In addition rapid accumulation of CD8 IFNγ-secreting cells in the lungs after challenge correlates with BCG-induced protection (2).
Taken together these data indicate that although CD4 Th1 cells and IFNγ are important components of protection against M. tuberculosis, other immune mechanisms can contribute to increased protection. Recently multi-functional CD4 T cells secreting IFNγ, TNF and IL-2, have been proposed as one such component and have been shown to correlate with protection in Leishmania major infection in mice (13). Furthermore a high frequency of PPD-specific multifunctional cells has been demonstrated in BCG vaccinated mice and humans, although there is no direct evidence that they provide protection against M. tuberculosis (13).
In the experiments described here, we took advantage of the observation that boosting BCG-primed mice with Ad85A by different routes has very different effects on protection against aerosol challenge with M. tuberculosis. We used these contrasting immunization regimes to determine what features of the immune response induced by boosting correlate with protection over and above that afforded by BCG immunization alone. We show that the presence of large numbers of multifunctional T cells in the lung, but not spleen, correlates with a reduction in mycobacterial burden after M. tuberculosis aerosol challenge.
Materials Methods
Animals and immunizations
All experiments were performed with 6-8 week old female BALB/c mice (Harlan Orlac, Blackthorn, UK), were approved by the animal use ethical committee of Oxford University and fully complied with the relevant Home Office guidelines. BCG (Pasteur 1173P2 D2 kindly provided by Dr Lagranderie, Pasteur Institute, France) was administered subcutaneously in the left hind foot pad at a dose of 2 × 105 colony forming units (CFU) in 30 ml volume.
The construction, design and preparation of Human adenovirus type 5 (AdHu5) E1/E3 deleted, expressing M. tuberculosis Ag85A (Ad85A), using the human CMV promoter containing intron A, is described in (14). The construct was generated by the group of Dr J Wilson, University of Pennsylvania School of Medicine, PA, USA. In some experiments mice were immunized with an empty control adenovirus, consisting of a modified Gateway entry vector pENTR4.CMV.BGH cloned into an AdHu5 destination vector pAd-PL DEST (Invitrogen, Paisley, UK).
Ten weeks after the BCG prime the Ad85A booster immunisation was administered either intradermally or intranasally. Mice were anesthetized and immunized intradermally with 25 μl in each ear (total 50 μl containing 2 × 109 vp of Ad85A per mouse) or for the intranasal administration allowed slowly to inhale 50 μl of 2 × 109 vp of Ad85A.
M. tuberculosis aerosol challenge
Four weeks after the Ad85A booster immunization, mice were challenged by aerosol with M. tuberculosis (Erdman strain, CBER/FDA, Maryland, USA), using a modified Henderson apparatus (15). Deposition in the lungs was measured 24 h after challenge and was ~200 CFU/lung. Mice were sacrificed 4 (one experiment) or 6 (two experiments) weeks after M. tuberculosis challenge. Spleens and lungs were homogenized and the bacterial load was determined by plating 10-fold serial dilutions of tissue homogenates on Middlebrook 7H11 agar plates (E & O Laboratories Ltd, Bonnybridge, UK). Colonies were counted after 3-4 weeks of incubation at 37°C.
Cell isolations and peptides
Spleen and lung cells were cultured in α-MEM supplemented with 10% heat inactivated FBS, L-glutamine, 2-mercapthoethanol, penicillin and streptomycin. Lungs were perfused with PBS via the right ventricle, cut into small pieces and digested with 0.7 mg/ml collagenase type I (Sigma) and 30 μg/ml DNase I (Sigma) for 45 min at 37°C. Lung fragments were then crushed through a 70-mm Falcon strainer using a 5 ml syringe plunger, washed and enumerated.
Cells were stimulated with PPD at 20 μg/ml (SSI, Copenhagen, Denmark) or a pool of sixty six 15mer peptides overlapping by 10 amino acids and covering the entire sequence of 85A. Each peptide was at a final concentration of 8 μg/ml during the stimulation. In some experiments cells were stimulated with the CD4 (Ag85A99-118aa TFLTSELPGWLQANRHVKPT) and CD8 (Ag85A70-78aa MPVGGQSSF) dominant peptide epitopes of antigen 85A at 2 μg/ml. Peptides were synthesized by Peptide Protein Research Ltd, Fareham, UK.
Flow cytometry
Cells harvested from spleen, lungs or blood were stimulated with PPD or pooled 85A peptides for 1 hour at 37°C. Golgi Plug (BD Biosciences) was added according to the manufacturer's instruction and cells were incubated for an additional 5 hours before intracellular cytokine staining. Cells were washed and incubated with CD16/CD32 mAB to block Fc binding. Subsequently the cells were stained for CD4, CD8, α4 intergin chain and IFNγ, IL-2 and TNF (eBioscience) using the BD Cytofix/Cytoperm kit according to the manufacturer's instructions. Cells were fixed with PBS 1% paraformaldehyde, run on a CyAN ADP Analyzer (Dako, Ely, UK) and analysed using FlowJo software. Median fluorescence indices (MFIs) and integrated MFIs (iMFIs) were calculated using Spice 4.1.6, kindly provided by Dr M. Roederer, Vaccine Research Centre, NIAID, NIH, USA. In some experiments antigen specific CD8 cells were enumerated using H-2Ld 85A peptide 70-78aa (MPVGGQSSF) tetramers (supplied by the NIH Tetramer Facility, NIAID, Bethesda, USA), the dominant 85A epitope in H-2D mice.
Three individual mice were analysed per group. Cytokine frequencies and numbers presented are after background subtraction of identically gated population of cells from the same sample, incubated with medium only.
ELISPOT and CBA assays
ELISPOT IFNγ assay was carried out as previously described (4) using coating and detecting antibodies from Mabtech Ab, Sweden. Spleen and lung cells were assayed following 18-20h stimulation with PPD, individual peptides or pool of peptides. Three individual mice were tested in each group and each condition was tested in duplicate.
For Cytometric Bead Array assay (CBA, BD Biosciences) 1 × 107 splenocytes were stimulated for 18h with 20 μg/ml PPD or 8 μg/ml pooled 85A peptides. The level of cytokines in the supernatant was measured using mouse inflammation and Th1/Th2 CBA kits, following the manufacturer's instructions. Three individual mice were tested for each group. Controls with cells cultured in medium alone were included and the background cytokine production from these cultures subtracted.
Mycobacterial growth inhibition assay
The assay for the ability of lung and splenic cells to inhibit the intracellular growth of BCG was adapted from Hoft et al.(16). Briefly lung and splenic cells from BA i.d and BA i.n. immunized mice were isolated as described above and plated in 6 well plates at a concentration of 5 × 105/ml in αMEM, 10% FBS. They were cultured in medium alone or with PPD or recombinant antigen 85A at 20μg/ml for 6 days.
Peritoneal macrophages were obtained from BALB/c mice plated at 15,000 cells/well in U bottom 96 well microtiter in αMEM and incubated at 37°C overnight. The next day non-adherent cells were removed and the adherent cells were infected with BCG for 24 overnight at a MOI of 5:1. The infected cells were then washed three times with warm antibiotic-free medium to remove extracellular BCG and the stimulated splenic or lung cells were added at an effector to target ratio of 10:1. After 72 hours co-culture, 0.2% saponin was added for 1 hour to release BCG from the infected macrophages and viable organisms were quantitated by plating on 7H11 agar. Results are expressed as % inhibition of mycobacterial growth calculated by the formula, C-E/C x100, where C is the CFU in co-cultures of infected macrophages with T cells rested for 5 days in medium alone and E is CFU in co-cultures containing T cells stimulated with antigen.
Statistical analysis
All results are representative of at least two independent experiments with similar results. Data were analysed using the non parametric Mann-Whitney test.
Results
Intranasal but not intradermal boosting with Ad85A improves protection afforded by BCG
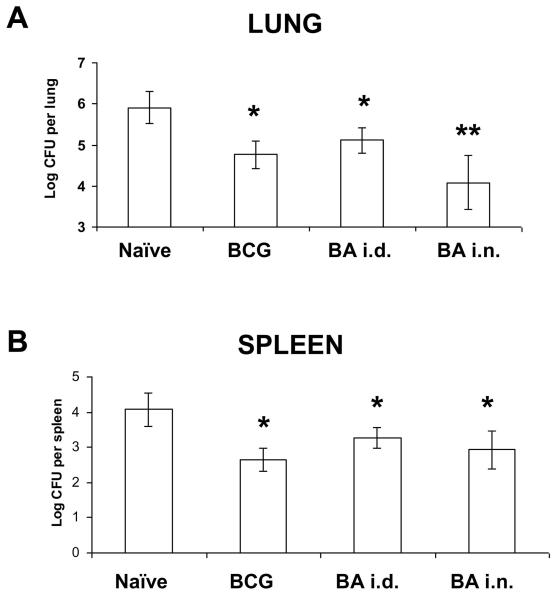
We used a human adenovirus type 5 containing antigen 85A (Ad85A), to boost immunity in mice following BCG priming. The boost was administered either intranasally (i.n., BA i.n. immunization) or intradermally (i.d., BAi.d. immunization) 10 weeks after BCG. Mycobacterial CFU were enumerated at 6 weeks after M. tuberculosis aerosol challenge. Intranasal but not i.d. boosting reduced mycobacterial CFU in the lungs compared to BCG alone (Fig. 1A). This is consistent with previous studies comparing parenteral (intramuscular) and intranasal administration of another adenovirus vector expressing 85A (9, 17). In the spleen BA i.d. and BA i.n. give equivalent CFU to BCG (Fig. 1B).
Figure 1. Mycobacterial CFU after Ad85A boosting of BCG primed mice.
BALB/c mice were immunised with BCG and 10 weeks later boosted with Ad85A given intradermally (BA i.d.) or intranasally (BA i.n.). Mice were challenged with M. tuberculosis by aerosol 4 weeks after the boost. Mice were sacrificed 6 weeks later and lungs and spleen CFU enumerated. Data represent the mean +/− SD from 6-8 mice per group. * p<0.05 versus naïve, **p<0.05 versus BCG and BA i.d. Similar results were obtained in two other experiments one harvested at 4 weeks and another at 6 weeks.
Spleen responses
Having identified two boosting regimes with very different outcomes, we next asked what are the hallmarks of protective and non-protective responses to booster immunizations. Because it has been suggested that cells simultaneously producing IFNγ, IL-2 and TNF may provide optimal effector function and protection against another intracellular pathogen (13) we defined the proportions of multifunctional cells in our immunized animals.
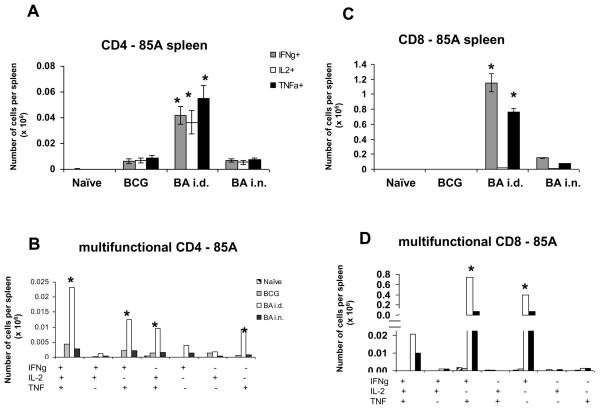
We performed intracellular staining for IFNγ, IL-2 and TNF on splenocytes stimulated with a pool of peptides covering the whole of antigen 85A. The BA i.d. regime induced the strongest IFNγ, TNF and IL-2 responses by CD4 cells (~ 50,000 or 0.2% antigen specific cells) almost 5 times greater than in BCG or BA i.n. immunized mice (Fig. 2A). The pattern of multifunctional cytokine producing cells is similar for all regimes, with the highest proportion being 3+ cells producing IFNγ, IL-2 and TNF, followed by 2+ (IFNγ+TNF+ and IL-2+TNF+) and 1+ (TNF+) (Fig. 2B). There is no difference between the BCG and BA i.n. CD4 response.
Figure 2. Cytokine responses of splenic T cells to antigen 85A.
Mice were primed with BCG and boosted with Ad85A either intradermally (BA i.d) or intranasally (BA i.n.). Splenocytes were isolated 4 weeks after the boost and stimulated with pooled 85A peptides for 6 hours. The frequencies of IFNγ, IL-2 and TNF producing cells were determined by flow cytometry on CD4 (A) or CD8 (C) gated cells and total number of cells per organ calculated. The number of cells expressing each of the seven possible combinations of the cytokines is also shown for CD4 (B) and CD8 (D) cells. Results are expressed as the mean +/− SEM of three mice per group, representative of two independent experiments. * p< 0.05 compared to B and BA i.n.
BA i.d. induced the strongest CD8 response (~1.1×106 or 10% antigen specific cells) followed by the BA i.n. regime (~1×105 cells) (Fig. 2C and D). The vast majority of the CD8 cells in the BA i.d. and BA i.n. mice are 2+ (IFNγ+TNF+) and 1+ (IFNγ+), because only 0.2% of the CD8 cells produce IL-2. Nevertheless in the BA i.d. group there are nearly as many 3+ CD8 as 3+ CD4 cells (~23,000 CD4 vs ~20,000 CD8 cells). The most effective BA i.n. regime induces approximately 3,000 CD4 3+ vs 10,000 CD8 3+ cells. BCG alone induces very few 85A specific CD8 cells.
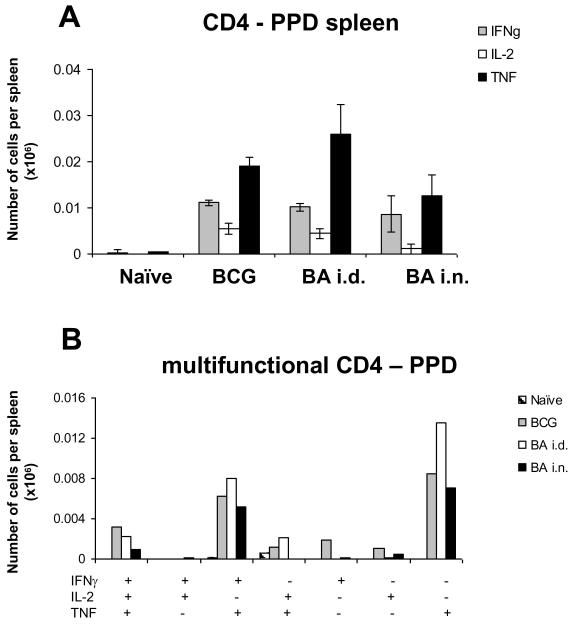
Following PPD stimulation in vitro no significant differences are detected in the CD4 responses between the regimes (Fig. 3A and B). The largest proportion of the CD4 responding cells produce TNF only, followed by 1+ (IFNγ+) and 3+ cells. This is not dissimilar from the data of Darrah et al (13), although they showed somewhat higher proportion of 3+ cells in a different mouse strain, C57Bl6. The pattern of cytokine production in response to PPD differs from that induced by antigen 85A probably because only the response to 85A has been boosted. Boosting changes the pattern and increases the proportion of 3+ cells. Very low number of CD8 cells responding to PPD are detected (less than 1,000 cytokine producing cells per spleen, data not shown).
Figure 3. Cytokine responses of splenic T cells to PPD.
Mice were primed with BCG and boosted with Ad85A either intradermally (BA i.d) or intranasally (BA i.n.). Splenocytes were isolated 4 weeks after the boost and stimulated with PPD for 6 hours. The frequencies of IFNγ, IL-2 and TNF producing cells were determined by flow cytometry on CD4 (A) gated cells and total numbers of cells per organ calculated. The number of cells expressing each of the seven possible combinations of the cytokines is shown for CD4 (B) cells. CD8 responses are very low and not shown. Results are expressed as the mean +/− SEM of three mice per group, representative of two independent experiments.
The magnitude and nature of responses in the spleen was also confirmed by the actual secretion of cytokines measured by CBA assay after 18 hours stimulation with 85A or PPD (Table). The BA i.d. regime induces more IFNγ, TNF, IL-2 and IL-6 following 85A stimulation than the other regimes. However following PPD stimulation although more IFNγ, TNF, IL-2 and IL-6 were produced, there are no significant differences between the regimes. No significant differences in MCP-1, IL-10, IL-4, IL-5, IL-12 production are detected after 85A or PPD stimulation (data not shown).
Table. Spleen cell PPD and antigen 85A specific cytokine responses.
| N | B | BA i.d. | BA i.n. | |
|---|---|---|---|---|
| 85A | ||||
| IFNγ | 13 | 70 | 1,653* | 435 |
| IL-2 | 5 | 12 | 157* | 52 |
| TNF | 24 | 53 | 189* | 51 |
| IL-6 | 7 | 11 | 123* | 39 |
| PPD | ||||
| IFNγ | 73 | 1,895 | 2,896 | 3,290 |
| IL-2 | 6 | 316 | 294 | 245 |
| TNF | 56 | 647 | 854 | 759 |
| IL-6 | 14 | 150 | 130 | 126 |
Mice were primed with BCG and boosted with Ad85A either intradermally (BA i.d) or intranasally (BA i.n.). Splenocytes were isolated 4 weeks after the boost and stimulated with PPD or pooled 85A peptides for 18 hours. IFNγ, IL-2, TNF and IL-6 production was determined using Cytometric Bead Array CBA assay. All data is expressed in pg/ml. Shown is the mean of three mice per group, representative of two independent experiments. Overall interassay and intrassay variation is less than 15%.
p< 0.05 compared to B and BA i.n..
Finally it is interesting to note that the 85A specific response differs very little in mice immunized with Ad85A alone or BCG primed Ad85A boosted mice (data not shown), suggesting that BCG does not prime a response to 85A efficiently in BALB/c mice. Furthermore the PPD response is not boosted by administration of Ad85A, yet 85A is a major component of PPD. A possible explanation is that the epitopes recognised by CD4 and CD8 cells of BALB/c mice are not present in commercially available PPD. Alternatively there may be competition for binding to antigen presenting cells from the numerous other peptides present in PPD during the in vitro restimulation.
Taken together these data show that the magnitude of the response to antigen 85A in spleen, measured either as number of IFNγ or multifunctional cells or secreted cytokines, does not correlate with protection assessed by enumeration of CFU. Conversely the response to PPD is unaltered by i.d. or i.n. boosting with Ad85A and yet these groups have different levels of protection.
Lung responses
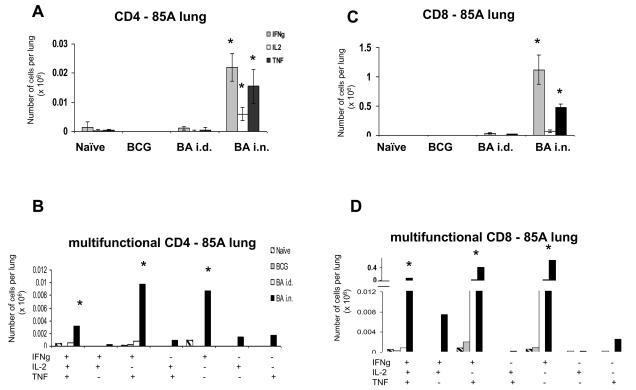
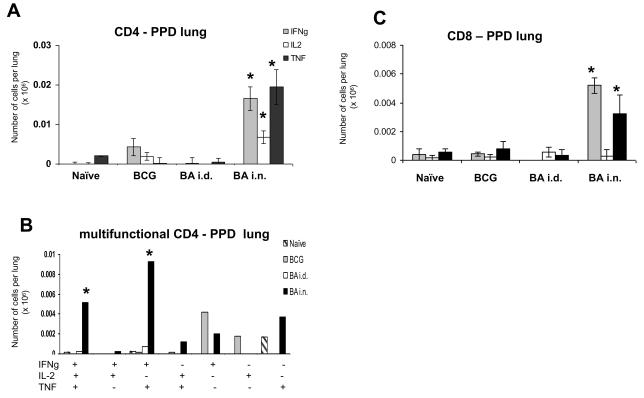
Lung responses differ from those in the spleen. The most effective BA i.n. regime induces very large CD4 and CD8 85A-specific responses (Fig. 4A). Nearly ~22,000 (0.5%) CD4 cells produce IFNγ. However less cells produce IL-2 compared to the spleen, so that most of the response is 2+ (IFNγ+TNF+), or 1+ (IFNγ+) and there are only ~3,000 3+ cells (Fig. 4B).
Figure 4. Cytokine responses of lung T cells to antigen 85A.
Mice were primed with BCG and boosted with Ad85A either intradermally (BA i.d) or intranasally (BA i.n.). Lung cells were isolated 4 weeks after the boost and stimulated with pooled 85A peptides for 6 hours. The frequencies of IFNγ, IL-2 and TNF producing cells were determined by flow cytometry on CD4 (A) or CD8 (C) gated cells and total numbers of cells per organ calculated. The number of cells expressing each of the seven possible combinations of the cytokines is shown for CD4 (B) and CD8 (D) cells. Results are expressed as the mean +/− SEM of three mice per group, representative of two independent experiments. * p< 0.05 compared to B and BA i.d.
There is a huge CD8 response in the BA i.n. group, with ~1.1×106 (~30%) of the CD8 cells producing cytokines. As in the spleen, a very low proportion of the antigen-specific CD8 cells produce IL-2 and therefore most of the cytokine producing cells are 2+ (IFNγ+TNF+) and 1+ (IFNγ+) producing cells. But nevertheless there are more 3+ CD8 than 3+ CD4 cells (~60,000 CD8 vs ~3,000 CD4) (Fig. 4C and D).
Whereas in the spleen i.n. or i.d. boosting does not alter the response to PPD, in the lung the BA i.n. regime increases the response considerably (Fig. 5).This may occur because circulating PPD specific CD4 memory cells are trapped in the lung due to the inflammation induced by Ad85A i.n. immunisation. However we did not see any non-specific trapping of antigen-specific CD8 tetramer-binding cells at 4 weeks after the boost, when control empty AdHu5 vector was administered i.n. at the time of i.d boosting with Ad85A (data not shown). However, non-specific trapping has been shown to be transient and CD4 and CD8 cells may behave differently (12). There are ~17,000 CD4 antigen specific cytokine producing cells, of which ~5,200 are 3+ cells and ~9,300 2+ (IFNγ+TNF+). The CD8 response to PPD, as in the spleen is weak, but Ad85A i.n. increases it significantly to ~5,000 CD8 IFNγ+ cells. Because the total number of PPD specific CD8 cells is low, we did not enumerate multifunctional cells.
Figure 5. Cytokine responses of lung T cells to PPD.
Mice were primed with BCG and boosted with Ad85A either intradermally (BA i.d) or intranasally (BA i.n.). Lung cells were isolated 4 weeks after the boost and stimulated with PPD for 6 hours. The frequencies of IFNγ, IL-2 and TNF producing cells were determined by flow cytometry on CD4 (A) or CD8 (C) gated cells and total numbers of cells per organ calculated. The number of cells expressing each of the seven possible combinations of the cytokines is shown only for CD4 (B) cells, but not for CD8 because the numbers are too low for reliable analysis. Results are expressed as the mean +/− SEM of three mice per group, representative of two independent experiments. * p< 0.05 compared to B and BA i.d.
Quality of responses
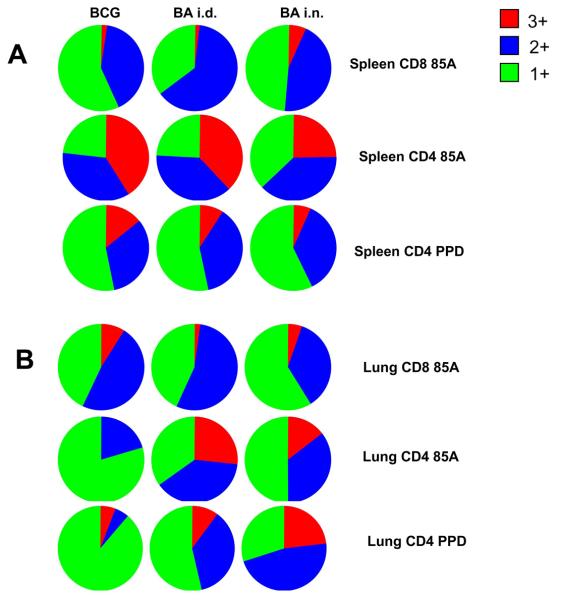
We further studied the quality of spleen and lung responses by computing the MFI of staining for intracellular cytokines (13). Figure 6A summarises the proportions of single, double and triple cytokine-producing cells in the spleen. Although the absolute numbers are very different (Figs. 2 and 3), the proportions of different cytokine-producing cells are very similar in all immunized mice. However, the large CD8 85A-specific population in the BA i.d. mice has the highest cytokine MFIs in the spleen, although it is the 2+ cells, which produce the highest level of cytokines. In contrast, among CD4 cells, 85A and PPD-specific cells from BCG immunized mice show the highest MFIs (data not shown). However when integrated MFIs are considered it is the BA i.d. regime that shows the largest IL-2, TNF and IFNγ production, in spite of the fact that this regime does not increase protection.
Figure 6. Multifunctional cytokine producing cells in the spleen and lungs.
Pie charts indicate the proportions of single (1+ green), dual (2+ blue) and triple (3+ red) producers of IFNγ, TNF and IL-2 in the spleen (A) and lungs (B) of immunized mice.
In contrast in the lung, the boosting regimes appear to induce different proportions of single or multiple cytokine-producing 85A-specific cells compared to BCG (Fig. 6B). However, BCG or BA i.d. immunization induces very few 85A specific CD4 or CD8 cells (Figs. 4 and 5), so that interpretation of these data requires caution. Nevertheless, it appears that BCG alone induces a higher proportion of single cytokine-producing CD4 cells, since not only the 85A-specific cells but PPD-specific CD4 cells from BCG immunized mice show a lower proportion of multiple cytokine-producing cells (Fig. 6B).
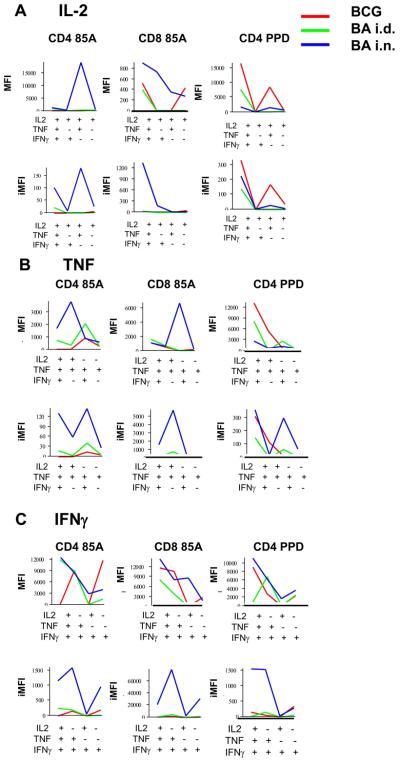
The MFIs of cytokine producing 85A-specific CD4 and CD8 cells induced by the BA i.n. regime are higher than those of 85A-specific cells induced by other regimes but it is not always the 3+ cells which produce the highest amount of cytokines (Fig. 7). However, the integrated MFIs for the 85A-specific cells in the BA i.n. regime are consistently the highest for all three cytokines, reflecting the large number of antigen specific lung cells found after this immunization (Fig. 7). Interestingly, the MFI for the CD4 PPD-specific response is highest for TNF and IL-2 in the BCG immunized mice with no clear pattern for IFNγ. The iMFI for IL-2 is also highest for CD4 PPD IL-2 producers in the BCG immunized mice.
Figure 7. MFIs and iMFIs for intracellular cytokines of lung CD4 and CD8 T cells of immunized mice.
MFIs indicate the intensity of staining for intracellular cytokines in single, dual or triple cytokine producing cells. Integrated MFIs (iMFIs) are the product of the intensity of staining and the number of cytokine producing cells. MFIs and iMFIs are shown for cells producing (A) IL-2, (B) TNF and (C) IFNγ from mice immunized with BCG alone (red line) BA i.d. (green line) or BA i.n. (blue line) and restimulated in vitro with either pooled antigen 85A peptides or PPD.
In summary, the most effective BA i.n. regime induces a strong lung CD4 and CD8 response to 85A and also boosts the CD4 response to PPD. These responses consist of cells with high amounts of intracellular cytokines (the 3+ cells are not always the highest), which may account for their protective efficacy. When both the amount cytokine and the number of cells are taken into account by calculating the iMFIs (13), the BA i.n. regime appears overwhelmingly the most potent in inducing a lung 85A-specific response.
The lung and spleen responses are relatively persistent
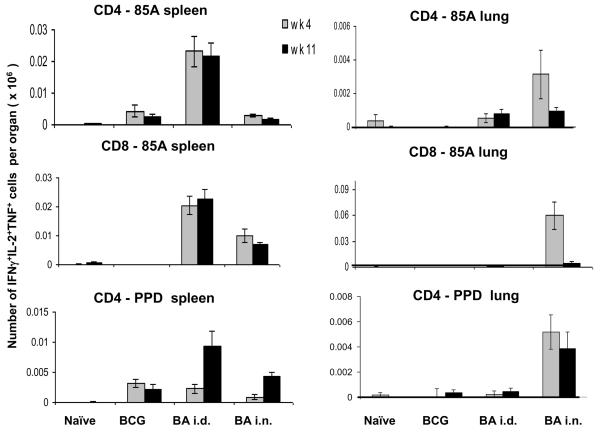
All of the immunogenicity data described above was performed at 4 weeks post boost as this is the time when the animals are challenged with M. tuberculosis. However if prime boost regimes are to be useful it will be important that they should induce persistent responses. We therefore assessed the immune response at a later time point, 11 weeks post boost. The number of multifunctional 3+ CD4 and CD8 cells responsive to 85A does not change in the spleen, while it is reduced in the lung (Fig. 8). In spite of the decrease of 85A specific cells in the lung at 11 wks, nevertheless ~1,000 CD4 and ~5,000 CD8 3+ cells remain. Interestingly the CD4 response to PPD is not changed in the lung and increased in the spleen at 11 wks BA i.d. and BA i.n. mice (Fig. 5). Overall the same trends were seen for the 2+ and 1+ producing cells (data not shown). These data show that an antigen specific (memory) T cell population persists in the BA i.d. and BA i.n. groups at 11 wks, although declining relatively rapidly in the lungs. Consistent with this, in a preliminary experiment we found that there is no difference in mycobacterial burden in the lungs between BCG and BA i.n. mice challenged 10 weeks after the boost (data not shown).
Figure 8. Multifunctional cells at 4 and 11 weeks after Ad85A boost.
The number of multifunctional 3+ cells expressing IFNγ, IL-2 and TNF in spleen (A) and lung (B) in response to pooled 85A peptides or PPD at 4 and 11 weeks post boost are shown.
Properties of antigen 85A specific cells
Antigen specificity and mycobacterial inhibitory capacity of cells
We confirmed using individual peptides and Elispot assays as well as tetramer staining for CD8 cells, that the CD4 and CD8 responses in spleen and lung show identical specificity for the dominant IAd-restricted 85A99-118aa peptide and H-2Ld85A70-78aa peptides, as reported by others (4, 17, 18) (data not shown). These results indicate that the reduction in CFU induced by i.n. immunization is not due to induction of T cells with differing antigen specificity to those induced by systemic immunization.
We next compared the ability of lung cells isolated from BA i.n. and spleen cells from BA i.d. immunized mice to inhibit mycobacterial growth in macrophages. Both lung cells (from BA i.n. mice) and spleen cells (from BA.i.d. mice), inhibit BCG growth to an equal extent, 45% at 10:1 effector to target ratio, indicating that both cell types have similar capacity to inhibit mycabacterial growth irrespective of their geographic location. However, the presence of inhibitory cells in the spleen does not correlate with effective immunity in the lung. This result has important implications for development of vaccines against M.tuberculosis.
Integrin expression
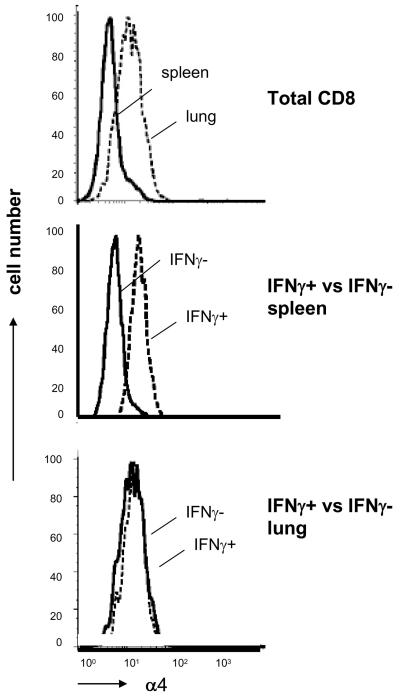
As the most effective BA i.n regime is associated with the presence of a very large number of cells in the lung but few in the spleen, we investigated what factors are important for the localization of the immune cells in the lung. It has been shown that VCAM-1, a vascular adhesion molecule, is upregulated on endothelium in inflamed lungs (19). The integrins α4β1 and α4β7 are the counter receptors on lymphocytes for VCAM-1 (20). We therefore studied the expression of the shared α4 chain on the CD8 T cells in the lung and spleen of Ad85A boosted mice. When total CD8 cells from lung and spleen are compared, those in the lung consistently express more α4, irrespective of the immunization of the mice (illustrated for BA i.n. mice in Fig. 9). However when expression of α4 is compared on IFNγ+ and IFNγ− cells, a different picture emerges. In the spleen, the IFNγ+ cells are consistently more strongly stained than IFNγ− cells, while in the lung both populations are equally strongly stained (Fig. 9). These data suggest that immmunization with Ad85A generates a population of splenic antigen-specific cells that have upregulated the α4 integrin chain and therefore have the potential to migrate efficiently to tissues that express the counter-receptors, VCAM-1 and MAdCAM (20). In contrast, in the lung both 85A-specific and other CD8 T cells express high levels of α4 suggesting that either all these cells have entered the lungs because they express appropriate addressins and adhesion molecules or that following entry or immunization in situ, lung resident cells upregulate the α4 chain (19, 21).
Figure 9. Expression of the alpha4 integrin chain on spleen and lung CD8 cells.
Expression of alpha 4 on CD8 gated T cells from spleen or lung (top panel), on intracellular IFNγ+ /− splenic CD8 cells stimulated in vitro with pooled 85A peptides (middle panel) and on IFNγ +/− lung CD8 cells (bottom panel). All cells are from BA i.n. immunized mice.
Discussion
Our data show that the magnitude of the immune response in the spleen does not correlate with reduction in lung mycobacterial CFU following aerosol challenge with M. tuberculosis in BALB/c mice primed with BCG and boosted with Ad85A intradermally or intranasally. The BA i.d. regime induces the largest splenic 85A-specific CD4 and CD8 cytokine response and yet does not reduce CFU compared to BCG alone. A similar lack of correlation between reduction in CFU and the magnitude of the splenic response has been shown following parenteral boosting with a different Ad85A construct (9), MVA85A (5) or recombinant antigen 85B (3). Similarly the magnitude of the blood cytokine response to 85A mirrors that for the spleen and does not correlate with CFU reduction either (data not shown).
Recently multifunctional Th1 cells were defined as a correlate of protection against Leishmania major (13). In the same study, multifunctional CD4 PPD-specific cells were detected in BCG vaccinated mice and humans, however there is no direct evidence that these are important for protection against M. tuberculosis (13). CD8 multifunctional cells have also been proposed as a correlate of an effective response in HIV, as individuals with better control of infection have an increased frequency of such cells (22). In our experiments, although there is a strong CD4 and CD8 splenic response after 85A stimulation in BA i.d. immunized animals, generating a large number of cytokine producing cells with a high proportion of double and triple cytokine producing cells (Figs. 2 and 6A), no decrease in mycobacterial burden is observed. Conversely after boosting BCG primed mice with Ad85A i.n., although there is no change in the magnitude or nature of the CD4 PPD-specific or 85A-specific and only a relatively small splenic CD8 T cell response, there is a reduction in mycobacterial burden against aerosol challenge. Thus our data show that neither the frequency of antigen specific cells nor the presence of multifunctional cells in the spleen (or blood) correlate with reduction of mycobacterial burden following aerosol challenge, in this prime boost mouse model.
In contrast, we show that the magnitude of the response in the lung following boosting, correlates well with a reduction in CFU compared to immunization with BCG alone. In the best-protected mice, boosted with Ad85A i.n., there is a large lung CD4 and CD8 response to antigen 85A as well as an increase in CD4 PPD-specific responses compared to mice immunized with BCG only (Figs. 4 and 5). It is striking that the number of IFNγ specific CD4 or CD8 cells in the lungs of the BA i.n. animals are of the same order of magnitude as the numbers induced in the spleen by the same immunogen given i.d. This is reflected in the presence of similar number of multifunctional cells in the two organs, although in the spleen there are equal numbers of CD4 and CD8 3+ cells while in the lung the majority are CD8. Intriguingly the two immunization regimes that are effective, BCG and BA i.n., both induce cells with high MFIs for intracellular cytokines, suggesting that the rate of production may be important for protective immunity (Fig. 7).
A further striking feature of the response to this Ad85A construct, as is the case for others, is the excess of CD8 over CD4 cells (17). In the lung there is an increased total number of CD8 T cells and a high proportion of these (up to 30%) are antigen specific. These data suggests that following Ad85A boosting, the CD8 cells, which include the largest number of multifunctional cells, play an important role in protection against aerosol challenge, consistent with other data (2). Most likely either CD4 or CD8 cells can mediate protection equally well after prime boost immunization, because both boosting with recombinant adenoviruses as shown here and by others (9), or recombinant vaccinia viruses that induce largely CD4 responses (4), can reduce lung mycobacterial burden if given by an appropriate route (intranasally).
There does not appear to be a major difference in antigen specificity or function between splenic and lung-resident cells induced by intradermal or intranasal boosting respectively. Both the CD4 and the CD8 responses are against the same dominant epitopes of antigen 85A, in line with other data in H-2d mice (4, 17, 18). Therefore the exact specificity of the cells does not provide an explanation why the cells in the lung protect, while the splenic cells do not. Although we have demonstrated subtle differences in the MFIs of cytokine producing cells from the spleen and lung in i.n. and i.d. immunized mice, there may be other differences in cytokines that we have not measured and that also contribute to successful protective immunity. For example IL-17 is known to be important for mycobacterial immunity (5, 23), while it is also possible that i.d. boosting induces T regs, which can interfere with effective anti-mycobacterial responses (3, 24).
However, since antigen-85A-specific splenic cells transferred intra-tracheally can protect (17) and lung lymphocytes from BA i.n. mice and splenic lymphocytes from BA i.d. mice reduce mycobacterial CFU in infected macrophages equally well, we consider that the key element in protection against aerosol challenge afforded by intranasal boosting with Ad85A, is the induction of a lung resident population of antigen specific cells, present at the time of challenge. We suggest that following M. tuberculosis challenge there is a balance between M. tuberculosis and the immune response. If there is a large immune population in the lung (such as that provided by i.n. immunisation) these cells immediately respond to the challenge organisms and can rapidly kill many of them. Abundant evidence indicates that tissue resident populations of T cells specific for other antigens have the properties of effector memory or activated effectors (21, 25, 26) and even central memory cells that enter the lung acquire effector memory phenotype and function (27). The lung cells in our BA i.n. immunized mice also show this phenotype (data not shown) and are therefore poised for effector function. Although purified T cells transferred into the airways cause a reduction in CFU after challenge (17), we cannot exclude the possibility that alterations in lung innate immune responses following i.n Ad85A boosting contribute to the observed reduction in lung CFU. Neither we nor others have yet performed challenge experiments after priming with BCG and boosting with an empty adenovirus (10, 12).
Following BCG or BA i.d. immunization a few antigen-specific cells will enter the lungs (Figs. 4 and 5) because some activated T cells can extravasate into nonlymphoid tissues (28-30). However the majority of spleen antigen-specific cells in BA i.d. boosted mice clearly do not enter the lungs even though they express high levels of the α4 integrin chain and the few lung-resident antigen-specific cells in BA i.d. immunized mice do not reduce mycobacterial burden beyond the level found in mice immunized with BCG alone. Thus we suggest that it is likely that the challenge organisms have to induce inflammation in order to upregulate VCAM-1 on lung vasculature and promote an influx of antigen-specific effector cells (19). Up-regulation of VCAM takes more than a week (18). By this time there will be more mycobacteria and some may have entered macrophages and deployed evasion strategies so that they are less readily killed. Studies of lung infiltrating lymphocytes post BCG challenge show that there are fewer cells in the lungs of BA i.d. than BA i.n. mice at 3 and 10 days post challenge (not shown), supporting the idea that the presence of cells in the lung at the time of, or very early after, challenge is critical for protective immunity (2).
Why is it that intranasal immunization induces such a large population of lung resident cells in the absence of a large systemic population? We suggest that the intranasal boost may have three effects. The first is induction of a local immune response in the upper respiratory nasal associated lymphoid tissue (NALT) (31) although it is not known whether this plays a role in protection against aerosol mycobacterial challenge (32). The second is that some cells primed in the NALT may home to the lung. Finally, it is very likely that much of the antigen is inhaled into the lung, reaching even the periphery of lung lobes, as has been shown for other antigens given intranasally in a volume of 50 microlitres (33). Once in the lung Ad85A induces a vigorous local immune response leading to an influx of immune cells into the airways (9, 12, 17).
Although these experiments leave many aspects of immunity to M. tuberculosis unresolved, they confirm the importance of lung-resident memory T cells (14). Protected lungs contain large numbers of multifunctional cytokine producing cells. Both PPD-specific and 85A-specifc lung cells from well protected animals have high levels of intracellular cytokines. Further experiments will be required to show whether this property is important for protection. The data have important implications for the design of human vaccines against M. tuberculosis first because they confirm data of many others, indicating that the magnitude and nature of immune responses measured in the spleen (or blood) do not correlate well with protection. Secondly they suggest that local immunity at the portal of entry is an important mechanism by which booster vaccines confer increased protection against mycobacterial infection. Challenges for the future will include the design of immunization regimes that can safely induce long lasting lung resident memory in humans and domestic animals and that can induce high level cytokine producing cells. There is also a pressing need to identify accessible, blood borne correlates of protection.
Abbreviations used in this paper
- M. tuberculosis
Mycobacterium tuberculosis
- BCG
Bacillus Calmette Guerin
- PPD
purified protein derivative
Footnotes
Disclosures
The authors have no financial conflict of interest.
REFERENCES
- 1.WHO/HTM/TB/2007.376.
- 2.Mittrucker HW, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann SH. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A. 2007;104:12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma C, Iona E, Giannoni F, Pardini M, Brunori L, Orefici G, Fattorini L, Cassone A. The Ag85B protein of Mycobacterium tuberculosis may turn a protective immune response induced by Ag85B-DNA vaccine into a potent but non-protective Th1 immune response in mice. Cell Microbiol. 2007;9:1455–1465. doi: 10.1111/j.1462-5822.2007.00884.x. [DOI] [PubMed] [Google Scholar]
- 4.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 5.Romano M, D'Souza S, Adnet PY, Laali R, Jurion F, Palfliet K, Huygen K. Priming but not boosting with plasmid DNA encoding mycolyltransferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine. 2006;24:3353–3364. doi: 10.1016/j.vaccine.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 6.Johnson L, Gough J, Spencer Y, Hewinson G, Vordermeier M, Wangoo A. Immunohistochemical markers augment evaluation of vaccine efficacy and disease severity in bacillus Calmette-Guerin (BCG) vaccinated cattle challenged with Mycobacterium bovis. Vet Immunol Immunopathol. 2006;111:219–229. doi: 10.1016/j.vetimm.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Santosuosso M, Ngai P, Zganiacz A, Xing Z. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J Immunol. 2004;173:4590–4597. doi: 10.4049/jimmunol.173.7.4590. [DOI] [PubMed] [Google Scholar]
- 8.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun. 2006;74:4634–4643. doi: 10.1128/IAI.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol. 2004;173:6357–6365. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- 11.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, Morris SL, Jacobs WR, Porcelli SA. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santosuosso M, McCormick S, Roediger E, Zhang X, Zganiacz A, Lichty BD, Xing Z. Mucosal luminal manipulation of T cell geography switches on protective efficacy by otherwise ineffective parenteral genetic immunization. J Immunol. 2007;178:2387–2395. doi: 10.4049/jimmunol.178.4.2387. [DOI] [PubMed] [Google Scholar]
- 13.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 14.Sridhar S, Reyes-Sandoval A, Draper S, Moore AC, Gilbert SC, Gao GP, Wilson JM, Hill AVS. Single dose protection against P. berghei by simian adenoviral vector using human CMV promoter containing intron A. J Virol. 2008;82:3822–3833. doi: 10.1128/JVI.02568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillpotts RJ, Brooks TJ, Cox CS. A simple device for the exposure of animals to infectious microorganisms by the airborne route. Epidemiol Infect. 1997;118:71–75. doi: 10.1017/s0950268896007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoft DF, Worku S, Kampmann B, Whalen CC, Ellner JJ, Hirsch CS, Brown RB, Larkin R, Li Q, Yun H, Silver RF. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis. 2002;186:1448–1457. doi: 10.1086/344359. [DOI] [PubMed] [Google Scholar]
- 17.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol. 2005;174:7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 18.Radosevic K, Wieland CW, Rodriguez A, Weverling GJ, Mintardjo R, Gillissen G, Vogels R, Skeiky YA, Hone DM, Sadoff JC, van der Poll T, Havenga M, Goudsmit J. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun. 2007;75:4105–4115. doi: 10.1128/IAI.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng CG, Britton WJ, Palendira U, Groat NL, Briscoe H, Bean AG. Up-regulation of VCAM-1 and differential expansion of beta integrin-expressing T lymphocytes are associated with immunity to pulmonary Mycobacterium tuberculosis infection. J Immunol. 2000;164:4853–4860. doi: 10.4049/jimmunol.164.9.4853. [DOI] [PubMed] [Google Scholar]
- 20.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 22.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 24.Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 25.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 26.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 29.Agrewala JN, Brown DM, Lepak NM, Duso D, Huston G, Swain SL. Unique ability of activated CD4+ T cells but not rested effectors to migrate to non-lymphoid sites in the absence of inflammation. J Biol Chem. 2007;282:6106–6115. doi: 10.1074/jbc.M608266200. [DOI] [PubMed] [Google Scholar]
- 30.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etchart N, Baaten B, Andersen SR, Hyland L, Wong SY, Hou S. Intranasal immunisation with inactivated RSV and bacterial adjuvants induces mucosal protection and abrogates eosinophilia upon challenge. Eur J Immunol. 2006;36:1136–1144. doi: 10.1002/eji.200535493. [DOI] [PubMed] [Google Scholar]
- 32.Cassidy JP, Bryson DG, Pollock JM, Evans RT, Forster F, Neill SD. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J Comp Pathol. 1998;119:27–44. doi: 10.1016/s0021-9975(98)80069-8. [DOI] [PubMed] [Google Scholar]
- 33.Minne A, Louahed J, Mehauden S, Baras B, Renauld JC, Vanbever R. The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response. Immunology. 2007;122:316–325. doi: 10.1111/j.1365-2567.2007.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]