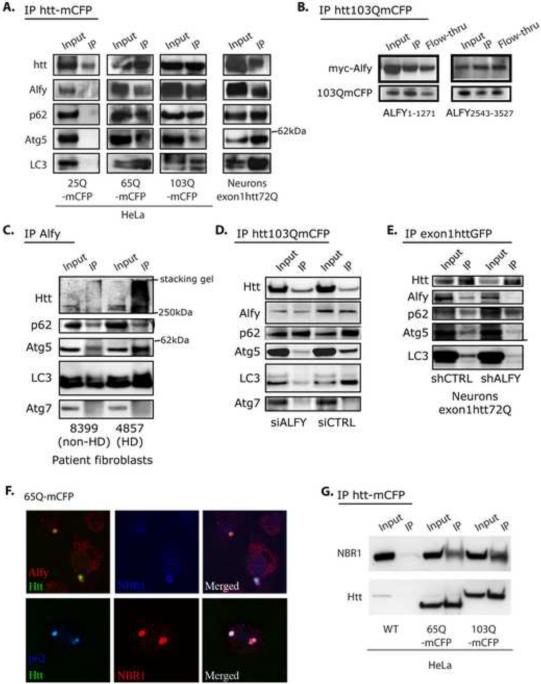
Figure 5.
Alfy is in a complex with the aggregation prone mutant huntingtin protein and endogenous Atg5, p62, NBR1 and LC3. A. Co-IP experiments from whole cell lysate from stable cell lines or primary neuronal model of HD. Lysates from stable cell lines Htt25Q-, Htt65Q- and Htt103Q-mCFP were first immunoprecipitated via the CFP-tag of the exon1Htt protein, then probed for endogenous proteins with antibodies against Alfy, p62, Atg5, Atg7 and LC3, and reprobed for Htt. Primary neurons transduced with lentivirus carrying exon1Htt72Q-GFP were first immunoprecipitated for Htt, then probed with antibodies against Alfy, p62 and LC3, and reprobed for Htt. B. Both a myc-tagged N-terminal and C-terminal fragment of Alfy co-IPs with exon1Htt-mCFP. 103QmCFP was IPed with an antibody against GFP which also recognizes the mCFP tag. C. Co-IP experiments with anti-Alfy serum from whole cell lysates from control or HD patient fibroblasts. In HD patient samples, Alfy co-IP with the mutant huntingtin protein, p62, Atg5 and LC3, but not Atg7. Alfy still co-IPs p62, Atg5 and LC3 in unaffected patient samples, suggesting that there are non-HD aggregates that may be recognized by Alfy. D–E. Alfy is required for bringing Atg5 and LC3 to the complex. Experiments were performed similarly to B, but after transfection with siRNA or shRNA against Alfy or a Ctrl sequence. D. Htt103Q-mCFP cell line. E. Primary rat cortical neurons. Immunoblotting (with antibodies against GFP, Alfy, p62, Atg5 and LC3) were all performed on the same blot. Based on the Htt input levels, transduction efficiency for 5F was not as efficient as in 5B. All IP experiments are representative images from at least 3 independent experiments. F. Endogenous Alfy, p62 and NBR1 all localize to 65Q-mCFP aggregates G. NBR1 forms a complex with the aggregation prone mutant huntingtin protein. Htt65Q- and Htt103Q-mCFP were IP'ed and the blot probed for NBR1