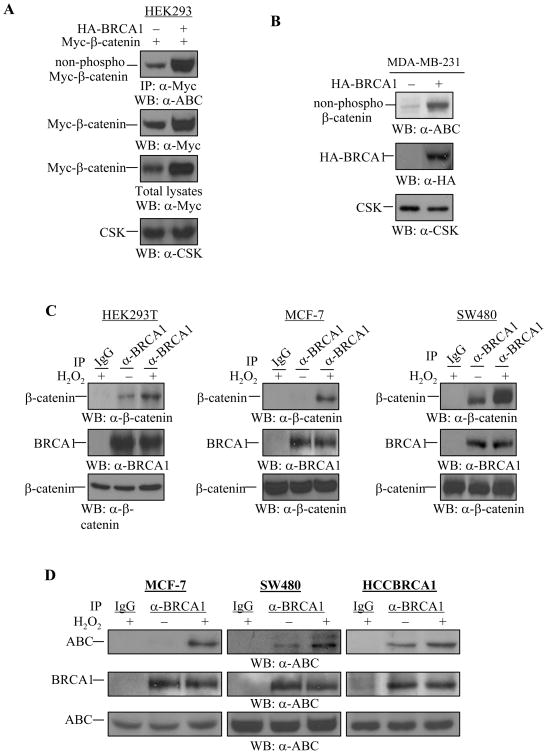
Figure 4. Regulation of Ser37/Thr41-non-phosphorylated β-catenin by WT-BRCA1 and H2O2.
(A) WT-BRCA1 increased the Ser37/Thr41-non-phosphorylated β-catenin protein expression in HEK293 cells. 0.375 μg of Myc-β-catenin plasmid was co-transfected with 0.375 μg of wild type BRCA1 into HEK293 cells. Myc-β-catenin protein was immunoprecipitated by anti-Myc antibody followed by Western blotting with anti-Ser37/Thr41-non-phosphorylated β-catenin (a-ABC) antibody. The membrane was reprobed with anti-Myc antibody to detect total Myc-β-catenin expression. 5 μg of total lysates were applied to detect Myc-β-catenin protein (total) expression, and anti-CSK antibody was used to monitor equal loading.
(B) WT-BRCA1 increased the expression of Ser37/Thr41-non-phosphorylated β-catenin in MDA-MB-231 cells. MDA-MB-231 cells were transfected with WT-BRCA1 or pcDNA3 vector (1.0 μg). 48 hrs after transfection, 20 μg of total cell lysates were applied for Western blotting to detect the Ser37/Thr41-non-phosphorylated β-catenin expression. Anti-CSK antibody was used to monitor equal loading. 20 μg of total lysates were used to confirm expression of the exogenous HA-BRCA1 protein by using anti-HA monoclonal antibody. The expression levels of ABC in HCC1937 cells and wild type BRCA1-reintroduced HCC1937 (HCC1937-BRCA1, Cl3.4). 25 μg of total lysates from HCC1937 and HCC1937-BRCA1, Cl3.4 were subjected to Western blotting with anti-ABC antibody. The same membrane was used to monitor equal loading with anti-Actin antibody. The levels of ABC in HCC1937 cells were lower than in HCC1937-BRCA1, Cl3.4.
(C) H2O2 induces BRCA1 and β-catenin protein association in vivo. HEK293T, MCF-7, and SW480 cells were untreated or treated with 100 μM of H2O2 for 1 hr. Equal amounts of total cell lysates were subjected to immunoprecipitation with anti-BRCA1 antibody, followed by Western blotting with anti-β-catenin antibody. The membrane was probed again with anti-BRCA1 polyclonal antibody. 5 μg of total lysates were used to monitor β-catenin protein expression.
(D) H2O2 induces BRCA1 and the active form of β-catenin protein association. MCF-7, SW480, and HCCBRCA1 cells were untreated or treated with 100 μM of H2O2 for 1 hr. Equal amounts of total cell lysates were subjected to immunoprecipitation with anti-BRCA1 antibody, followed by Western blotting with anti-ABC antibody. The membrane was probed again with anti-BRCA1 polyclonal antibody. 15 μg of total lysates were used to monitor ABC protein expression.