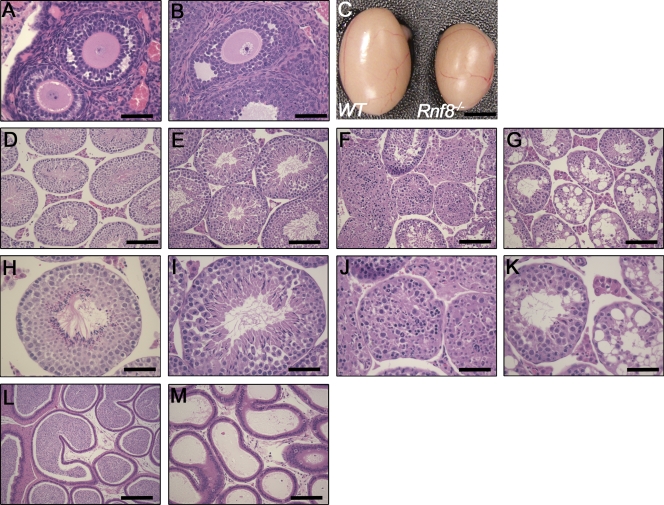
Figure 2.
Impaired spermatogenesis in Rnf8−/− mice. (A and B) Histological analysis of ovaries of WT (A) and Rnf8−/− (B) females showed no significant differences in ovarian architecture. Bars, 50 µm. (C) Testes from 12-mo-old Rnf8−/− male exhibited a significant reduction in size compared with those of WT littermates. Bar, 0.25 cm. (D–K) Images of H&E-stained seminiferous tubules from 1.5-mo-old WT (D and H) and Rnf8−/− (E, F, I, and J) mice and a 3.5-mo-old Rnf8−/− male (G and K). H&E staining of testes from Rnf8−/− males showed a spectrum of alterations in spermatogenesis. Rnf8−/− testis showed seminiferous tubules with well-populated layers of spermatogonia, spermatocytes, and mature spermatozoa (E and I) and other seminiferous tubules with only few or absent mature spermatozoa in the lumen and an unusually elevated number of immature spermatids and mitotic cells (E and I). Rnf8−/− testis showed disorganized epithelial architecture of seminiferous tubules, few spermatogonia and spermatocytes mixed together with apoptotic bodies, and the absence of mature sperm cells (F and J). Rnf8−/− testis showed focal vacuolar degeneration of most seminiferous tubules (G and K). In contrast to WT (L), H&E staining of epididymis of Rnf8−/− mice (M, 20X) show no histological abnormalities; however, they have a significant reduction of mature sperms. Bars: (D–G) 100 µm; (H–K) 50 µm; (L and M) 100 µm. A minimum of five mice per genotype have been analyzed.