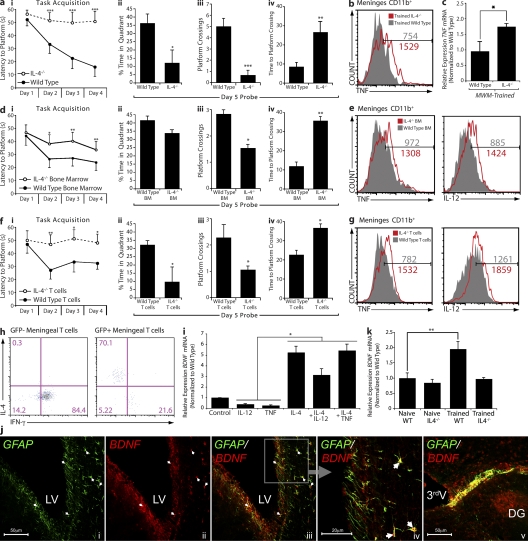
Figure 4.
T cell–derived IL-4 regulates meningeal myeloid cell phenotype and influences learning and memory. All behavior experiments were performed by an experimenter blinded to the identity of experimental groups and were recorded with the EthoVision video tracking system. Representative experiments are shown out of at least two to three independently performed in each case. (a, i–iv) Control and IL-4−/− mice of identical genetic background (C57BL/6J) were monitored during the MWM task performance. During the acquisition (i) phase of the task, IL-4−/− mice took significantly longer than controls to locate the hidden platform. Two-way repeated measures ANOVA was used for statistical analysis (***, P < 0.001). During the probe trial, IL-4−/− mice showed significantly reduced time spent in the training quadrant (ii), passed over the original platform location fewer times (iii), and took significantly longer to successfully locate the original platform location (iv; Student’s t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001). (b, e, and g) Meningeal isolates from mice were labeled for viability, CD45, singlet cells, and CD11b to allow exact comparison of cells (see Fig. 3 h for detailed gating strategy). Cells were also labeled for IL-12 and/or TNF expression. Histograms represent comparative proinflammatory cytokine production of 3 × 103 viable, CD45hi, CD11b+ singlet cells from each group. Numbers on the histograms indicate actual cell counts. Meningeal isolates from MWM-trained wild-type and IL-4−/− mice demonstrate increased production of TNF by CD11b+ cells in IL-4−/− mice (b). FACS data shown are from one out of two independent experiments with similar results and are representative. (c) Hippocampal tissue from wild-type and IL-4−/− mice was compared by qRT-PCR for relative levels of TNF mRNA expression; hippocampal isolates from IL-4−/− mice displayed significantly higher levels of TNF mRNA. One-way ANOVA was used for statistical analysis (*, P < 0.05; n = 4 from each group; experiment was repeated two times). (d, i–iv) Lethally irradiated wild-type mice reconstituted with bone marrow from wild-type or IL-4−/− mice were monitored during the MWM task performance. During the acquisition (i) phase of the task, mice that had received IL-4−/− bone marrow took significantly longer than controls to locate the hidden platform. Two-way repeated measures ANOVA was used for statistical analysis (n = 7 in each group; *, P < 0.05; **, P < 0.01). During the probe trial, mice that had received IL-4−/− bone marrow passed over the original platform location significantly fewer times (iii) and took significantly longer to successfully locate the original platform location (iv; Student’s t test; *, P < 0.05; **, P < 0.01). (e) Meningeal isolates were prepared from each group (n = 4 pooled samples), labeled as described, and analyzed by FACS. CD11b+ cells from mice that received bone marrow from IL-4−/− mice demonstrated increased levels of IL-12 and TNF. (f, i–iv) IL-4−/− mice received i.p. injection of 2 × 106 T cells from wild-type mice (expressing GFP under the UBC promoter) or IL-4−/− mice, and were tested after 2 wk in the MWM. During the acquisition (i) phase of the task, mice that had received IL-4−/− T cells took significantly longer than wild-type T cell–injected mice to locate the hidden platform. Two-way repeated measures ANOVA was used for statistical analysis (n = 8 in each group; *, P < 0.05; **, P < 0.01). During the probe trial, mice that had received IL-4−/− T cells showed significantly reduced time spent in the training quadrant (ii), passed over the original platform location significantly fewer times (iii), and took significantly longer to successfully locate the original platform location as compared with mice receiving T cells from wild-type donors (iv; Student’s t test; *, P < 0.05). (g) Meningeal isolates were prepared from each group (n = 4 pooled samples), labeled as described, and analyzed by FACS. CD11b+ cells from mice that received IL-4−/− T cells mice demonstrated increased levels of IL-12 and TNF compared with wild-type T cell–injected mice. (h) Meningeal isolates from IL-4−/− mice that received GFP+ T cells were also labeled extracellularly for CD3 and CD4, and intracellularly for IFN-γ and IL-4. GFP+ (wild-type, transferred) and GFP− (endogenous) CD4+ T cells were compared for expression of intracellular cytokines. GFP+ T cells expressed high levels of IL-4 and low levels of IFN-γ, whereas GFP− T cells expressed no IL-4 and high levels of IFN-γ. Numbers indicate percentages. (i) Primary cultures of astrocytes from wild-type mice were treated with 10 ng/ml of various recombinant cytokines. After 8 h, mRNA was isolated from astrocyte cultures and analyzed by qRT-PCR. Relative expression of BDNF mRNA under different treatment conditions is presented. One-way ANOVA was used for statistical analysis (*, P < 0.05). One representative experiment out of two independently performed experiments is presented. (j) Representative confocal images of astrocytes (glial fibrillary acidic protein; GFAP) lining the borders of the brain expressing BDNF are presented. Arrows indicate examples for areas of colocalization. DG, dentate gyrus; LV, lateral ventricle; 3rdV, third ventricle. (k) Hippocampi from naive and MWM-trained wild-type and IL-4−/− mice were examined for BDNF expression using qRT-PCR. Relative levels of BDNF mRNA expression are presented. One-way ANOVA was used for statistical analysis (**, P < 0.01; n = 4 from each group). One representative experiment out of three independently performed experiments is presented. Error bars represent SEM.